Mortality within three months after nonfatal ischemic stroke treated by mechanical thrombectomy in routine care-data from the German Stroke Registry
- PMID: 41035079
- PMCID: PMC12490031
- DOI: 10.1186/s42466-025-00427-7
Mortality within three months after nonfatal ischemic stroke treated by mechanical thrombectomy in routine care-data from the German Stroke Registry
Abstract
Background: Mechanical thrombectomy (MT) is a highly effective treatment for large vessel occlusion (LVO) ischemic stroke. However, a substantial share of patients have lethal outcome within 3 months. Individualization of outcome prognostication is needed to support clinical decision-making throughout the care pathway after MT. We investigate predictors of lethal outcome in patients with nonfatal LVO, defined by discharge alive from primary treating hospital, in a large prospective registry study of MT under routine care conditions.
Methods: 6,518 patients with nonfatal LVO treated by MT enrolled in the German Stroke Registry-Endovascular Treatment from May 2015-December 2021 were analysed with regard to lethal outcome by 3 month follow-up. Univariate group comparisons and multiple logistic regression analysis were performed to identify patients with high odds for survival or lethal outcome.
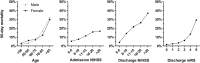
Results: We report 11.6% (757/6,518) 3 month mortality following hospital discharge after LVO treated by MT. Besides better functional outcome at discharge (modified Rankin scale < 4, odds ratio, OR [95% confidence interval, CI]: 2.38 [1.71-3.32], p < 0.001; National Institute of Health Stroke scale < 8, OR [95%CI]: 3.45 [2.55-4.66], p < 0.001), intravenous thrombolysis (OR [95%CI]: 1.48 [1.17-1.88], p = 0.001), successful recanalization (OR [95%CI]: 1.43 [1.08-1.90], p = 0.014) and discharge to a neurorehabilitative facility (versus nursing home: OR [95%CI]: 0.39 [0.26-0.58], p < 0.001; versus home: OR [95%CI]: 0.69 [0.49-0.97], p = 0.032) were independent predictors of survival. Predictors of lethal outcome were older age (OR [95%CI]: 1.09 [1.07-1.10], p < 0.001), male sex (OR [95%CI]: 1.24 [1.00-1.55], p = 0.049), premorbid disability (OR [95%CI]: 1.47 [1.08-2.02], p = 0.016), active smoking (OR [95%CI]: 1.51 [1.06-2.14], p = 0.023), anticoagulation therapy prior to LVO (OR [95%CI]: 1.45 [1.09-1.92], p = 0.010), stroke etiology, general anaesthesia during MT (OR [95%CI]: 1.31 [1.02-1.69], p = 0.035) and intracerebral haemorrhage (OR [95%CI]: 1.50 [1.13-1.99], p = 0.005).
Conclusions: Lethal outcome after hospital discharge within 3 months after MT is frequent, accounting for more than one quarter of overall 3-month mortality after MT of LVO. Predictors of survival enable individual outcome prognostication, which assists clinical decision-making with regard to surveillance concerning complications, rehabilitative resource allocation and counselling about goals of care.
Trial registration: ClinicalTrials.gov (Identifier: NCT03356392, Date of registration: 2017/11/22).
Keywords: Complications; Endovascular stroke therapy; Ischemic stroke; Mechanical thrombectomy; Mortality; Registry studies.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Study protocols and procedures were conducted in compliance with the Declaration of Helsinki. The German Stroke Registry–Endovascular Treatment (GSR-ET) is registered at ClinicalTrials.gov (Identifier: NCT03356392, date of registration: 2017/11/22) and was approved by the ethics committee of the leading center (Ludwig-Maximilians University Munich, protocol number: 689–15) and by the local ethics committees. Written informed consent was obtained from all participants (or guardians of participants). Consent for publication: Not applicable. Competing interests: AEO: speakers bureau and research grants from Canon medical. KG: personal fees and/or non-financial support from Abbott Medical, Boehringer Ingelheim, Bristol-Meyers Squibb and Pfizer Pharma GmbH. TU: personal fees from Merck Serono and Pfizer, grants from Else Kröner-Fresenius Stiftung. All other authors report no disclosures relevant to the manuscript.
Figures


References
-
- Alegiani, A. C., Dorn, F., Herzberg, M., Wollenweber, F. A., Kellert, L., Siebert, E., Nolte, C. H., Rennenberg, R., Hattingen, E., Petzold, G. C., Bode, F. J., Pfeilschifter, W., Schäfer, J. H., Wagner, M., Röther, J., Eckert, B., Kraft, P., Pham, M., Boeckh-Behrens, T., & Thomalla, G. (2019). Systematic evaluation of stroke thrombectomy in clinical practice: The German stroke registry endovascular treatment. International Journal of Stroke,14(4), 372–380. 10.1177/1747493018806199 - PubMed
-
- Burton, C. R., Payne, S., Addington-Hall, J., & Jones, A. (2010). The palliative care needs of acute stroke patients: A prospective study of hospital admissions. Age and Ageing,39(5), 554–559. 10.1093/ageing/afq077 - PubMed
-
- Campbell, D., Butler, E., Campbell, R. B., Ho, J., & Barber, P. A. (2023). General anesthesia compared with non-GA in endovascular thrombectomy for ischemic stroke: A systematic review and meta-analysis of randomized controlled trials. Neurology,100(16), e1655–e1663. 10.1212/WNL.0000000000207066 - PMC - PubMed
-
- Chabanne, R., Geeraerts, T., Begard, M., Balança, B., Rapido, F., Degos, V., Tavernier, B., Molliex, S., Velly, L., Verdonk, F., Lukaszewicz, A.-C., Perrigault, P.-F., Albucher, J.-F., Cognard, C., Guyot, A., Fernandez, C., Masgrau, A., Moreno, R., Ferrier, A., & Futier, E. (2023). Outcomes after endovascular therapy with procedural sedation vs general anesthesia in patients with acute ischemic stroke: The AMETIS randomized clinical trial. JAMA Neurology,80(5), 474–483. 10.1001/jamaneurol.2023.0413 - PMC - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
