High-intensity interval training improves cognitive dysfunction in chronically stressed mice through alleviating homocysteine-induced transcriptional repression of Cldn5
- PMID: 41035457
- PMCID: PMC12481931
- DOI: 10.1016/j.ynstr.2025.100758
High-intensity interval training improves cognitive dysfunction in chronically stressed mice through alleviating homocysteine-induced transcriptional repression of Cldn5
Abstract
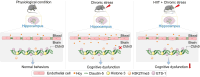
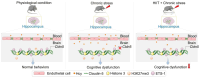
Chronic stress-induced blood-brain barrier (BBB) dysfunction contributes to neurological disorders, with homocysteine (HCY) as a key risk factor. Considering pharmacotherapy limitations, non-invasive interventions like high-intensity interval training (HIIT) are promising. To determine whether HIIT improves stress-induced BBB dysfunction and cognitive impairment, we first established a chronic unpredictable mild stress (CUMS) model and assigned mice into four groups: Control (Ctrl), CUMS, HIIT, and HIIT + CUMS. Here, we found that HIIT significantly ameliorated cognitive impairment in male CUMS mice, as evidenced by reduced escape latency in morris water maze and increased memory performance in novel object recognition test. HIIT also preserved BBB integrity by ameliorating the tight junction disruption and BBB hyper-permeability in stressed mice. Subsequently, to clarify the role of HCY in the HIIT-mediated effects, we established an HHCY model and divided mice into four groups: Ctrl, HIIT, HHCY, and HIIT + HHCY. The results showed that HIIT normalized the plasma and hippocampal HCY levels by restoring the expression of related metabolic enzymes including CBS, MTHFR and MS, and alleviated HHCY-induced cognitive decline and BBB damage. Further, HIIT reversed HCY-induced Claudin-5 downregulation by inhibiting H3K27me3 enrichment at the Cldn5 (the encoding gene of Claudin-5) promoter region. In addition, HIIT restored the expression of ETS1, one of the transcriptional activators of Cldn5, to facilitate the transcription of Cldn5 gene and the stabilization of BBB. Collectively, these findings reveal that HIIT improves chronic stress-induced cognitive impairment via eliminating the disruptive effects of HHCY on the BBB integrity, offering a non-pharmacological intervention potential for stress-related cognitive deficits.
Keywords: BBB damage; Cldn5 transcriptional repression; HCY; HIIT; Stress-induced cognitive dysfunction.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






References
LinkOut - more resources
Full Text Sources
Miscellaneous

