Design, development, and evaluation of gene therapeutics specific to KSHV-associated diseases
- PMID: 41035779
- PMCID: PMC12481920
- DOI: 10.1016/j.omton.2025.201050
Design, development, and evaluation of gene therapeutics specific to KSHV-associated diseases
Abstract
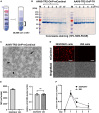
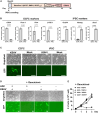
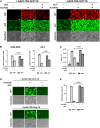
Kaposi sarcoma associated herpesvirus (KSHV) is the causative agent of Kaposi sarcoma (KS) and two human B cell lymphoproliferative diseases. KSHV-encoded latency-associated nuclear antigen (LANA) is expressed in KSHV-infected cancer cells. Thus, LANA is an attractive target for therapeutic intervention for KSHV-associated diseases. Here, we devised a cancer gene therapy vector using the adeno-associated virus (AAV), which capitalizes on the LANA's function to maintain terminal repeat (TR)-containing circular genomes and the TR's enhancer function for KSHV-inducible gene promoters. By including two TR copies with a lytic inducible gene promoter (TR2-OriP), we prepared an AAV vector, which expresses an engineered thymidine kinase (TK) selectively in KSHV-infected cells. Ganciclovir (GCV), an anti-herpesvirus drug, effectively eradicated multiple KSHV-infected cells that include induced pluripotent stem cell-derived epithelial colony-forming cells but not non-KSHV-infected counterparts in the presence of AAV8-TR2-OriP-TK. In addition, AAV8-TR2-OriP-TK prevents KSHV from producing virions from reactivated cells. Anti-cancer drugs, known to reactivate KSHV, stimulated TK expression from the vector and, therefore, synergized with AAV8-TR2-OriP-TK/GCV. Finally, the AAV8-TR2-OriP-TK/GCV effectively suppressed the growth of KSHV-infected cancer cells in the xenograft tumor model, whereas systemic intravenous AAV8-TR2-OriP-TK injection/GCV showed no detectable side effects. Our proof-of-concept studies highlight a promising strategy for targeting cancers driven by herpesviruses.
Keywords: Kaposi's sarcoma; Kaposi’s sarcoma-associated herpesvirus; MT: Regular Issue; adeno-associated virus; cancer gene therapy; oncogenic herpesvirus; oncolytic therapy; promoter-enhancer interaction; terminal repeats.
© 2025 The Authors.
Conflict of interest statement
Y.I. filed provisional patents related to vector design and utilization for therapeutics purposes through the University of California Davis and is a founder of VGN Bio, Inc.
Figures






Update of
-
Design, development, and evaluation of gene therapeutics specific to KSHV-associated diseases.bioRxiv [Preprint]. 2025 Feb 19:2025.02.19.639178. doi: 10.1101/2025.02.19.639178. bioRxiv. 2025. Update in: Mol Ther Oncol. 2025 Sep 05;33(4):201050. doi: 10.1016/j.omton.2025.201050. PMID: 40027700 Free PMC article. Updated. Preprint.
References
-
- Nador R.G., Cesarman E., Chadburn A., Dawson D.B., Ansari M.Q., Sald J., Knowles D.M. Primary effusion lymphoma: a distinct clinicopathologic entity associated with the Kaposi's sarcoma-associated herpes virus. Blood. 1996;88:645–656. - PubMed
-
- Soulier J., Grollet L., Oksenhendler E., Cacoub P., Cazals-Hatem D., Babinet P., d'Agay M.F., Clauvel J.P., Raphael M., Degos L., et al. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood. 1995;86:1276–1280. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
