Solving the light chain mismatch of IgG-like bispecific antibody by utilizing 2A peptide based FaBody platform
- PMID: 41036080
- PMCID: PMC12481933
- DOI: 10.1016/j.btre.2025.e00917
Solving the light chain mismatch of IgG-like bispecific antibody by utilizing 2A peptide based FaBody platform
Abstract
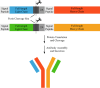
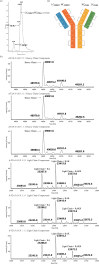
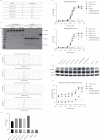
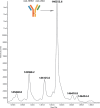
Bispecific antibodies (BsAbs), engineered to target multiple antigens or epitopes simultaneously, promise enhanced therapeutic efficacy over traditional monoclonal antibodies (mAbs). However, the complex BsAbs structure presents significant production challenges, particularly chain mismatch issues. This study presents a novel approach utilizing 2A peptides within a FaBody platform to address light chain mismatches in IgG-like BsAbs. By leveraging self-cleaving 2A peptides, stable expression in mammalian cells significantly improves the accuracy of antibody chain assembly. This strategy markedly enhances the production of correctly assembled IgG-like BsAbs, providing a promising solution to critical challenges in BsAbs drug development.
Keywords: 2A peptides; Bispecific antibodies; Fabody platform; Light chain mismatch.
© 2025 Published by Elsevier B.V.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






References
LinkOut - more resources
Full Text Sources
