Efficacy of Trastuzumab Deruxtecan in HER2-Positive and HER2-Low Metastatic Breast Cancer: A Real-World Retrospective Cohort Study in China
- PMID: 41036093
- PMCID: PMC12479221
- DOI: 10.2147/BCTT.S545308
Efficacy of Trastuzumab Deruxtecan in HER2-Positive and HER2-Low Metastatic Breast Cancer: A Real-World Retrospective Cohort Study in China
Abstract
Background: Limited real-world data are available on the effectiveness and safety of trastuzumab deruxtecan (T-DXd, DS8201a) in patients with HER2-positive and HER2-low metastatic breast cancer (MBC), particularly within the Chinese population.
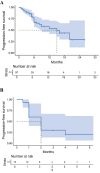
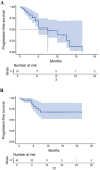
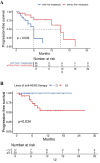
Methods: Between 2022 and 2025, 98 patients with MBC treated with T-DXd were retrospectively enrolled at Fudan University Shanghai Cancer Center. Patients were categorized as HER2-positive and HER2-low cohort. Clinical outcomes including objective response rate (ORR), progression-free survival (PFS), disease control rate (DCR), and clinical benefit rate (CBR), were assessed and compared between cohorts. The primary endpoint of the study was PFS, which was estimated using the Kaplan-Meier method and compared using the Log rank test.
Results: Among the 98 patients, the median PFS was 15.0 months. The ORR, DCR, and CBR were 48.0%, 69.4%, and 41.8%, respectively. HER2-positive patients experienced longer PFS compared to HER2-low patients (not reached vs 9.0 months). Among HER2-low patients, liver metastases were associated with poorer outcomes. Patients with brain metastases achieved a median PFS of 15.5 months and a 1-year PFS rate of 65.3%. Grade ≥3 adverse events included neutropenia (20.4%), nausea (5.1%), anemia (4.1%), and interstitial lung disease in 6.1% of patients, leading to discontinuation in 2.0%.
Conclusion: In this real-world analysis, T-DXd demonstrated robust clinical activity in both HER2-positive and HER2-low MBC, consistent with the findings from the DESTINY-Breast clinical trials. Notably, we identified several clinically relevant prognostic factors, including HER2 status, metastatic site, treatment line, and prior therapies. These findings support the broader clinical application of T-DXd and offer insights into individualized treatment selection.
Keywords: HER2; T-DXd; breast cancer; prognosis; real-world.
© 2025 Yan et al.
Conflict of interest statement
The authors declare that there is no conflict of interest in this work.
Figures
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

