Advances in tissue engineering for the repair of growth plate injuries
- PMID: 41036385
- PMCID: PMC12479520
- DOI: 10.3389/fbioe.2025.1608923
Advances in tissue engineering for the repair of growth plate injuries
Abstract
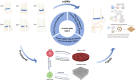
The growth plate is a cartilage tissue located between the epiphysis and diaphysis of long bones, responsible for the longitudinal growth of the skeleton. Due to its limited regenerative capacity, when the growth plate is damaged, it is typically replaced by inappropriate bone tissue, leading to the formation of bony bridges. These bony bridges not only restrict normal skeletal growth but may also cause limb length discrepancies, angular deformities, and functional impairments. Although traditional clinical treatments have shown some effectiveness, they are often associated with severe complications and poor prognoses. Therefore, the development of effective therapeutic strategies to prevent the formation of bony bridges and promote the repair and regeneration of the growth plate has become a current research focus. Cartilage tissue engineering, as an emerging therapeutic approach, restore the function of the growth plate through the substitution or repair of damaged cartilage tissue, has been widely applied in the repair of growth plate injuries. Cartilage tissue engineering for growth plate injury primarily relies on three key components: seed cells, growth factors, and scaffold materials. Seed cells provide the basis for cartilage regeneration, typically using autologous or allogeneic chondrocytes, mesenchymal stem cells, etc.,; growth factors such as bone morphogenetic proteins (BMPs) and transforming growth factor-beta (TGF-β) promote cell proliferation and differentiation, while regulating the synthesis of cartilage matrix; scaffold materials provide three-dimensional structural support, offering a platform for directed cell growth and tissue repair. In recent years, with continuous advancements in biomaterials and innovations in tissue engineering techniques, cartilage tissue engineering has shown promising prospects for application. This article systematically reviews the latest research progress on cartilage tissue engineering in the repair of growth plate injuries, based on a comprehensive search and analysis of relevant literature from databases such as PubMed and CNKI. The paper focuses on the classification and stages of growth plate injuries and discusses the three essential elements of tissue engineering treatment for growth plate injury.
Keywords: 3D printing technology; growth factors; growth plate injury; scaffold material; seed cells; tissue engineering.
Copyright © 2025 Wang, Zeng, Tu, Li, Xu and Zhuang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Adhikari B., Stager M. A., Collins E. G., Fischenich K. M., Olusoji J., Ruble A. F., et al. (2024). Sustained release of Mapk14-Targeting sirna from polyelectrolyte complex hydrogels mitigates msc osteogenesis in vitro with potential application in growth plate injury. J. Biomed. Mater Res. A 112 (12), 2346–2357. 10.1002/jbm.a.37784 - DOI - PubMed
-
- Asawa Y., Sakamoto T., Komura M., Watanabe M., Nishizawa S., Takazawa Y., et al. (2012). Early stage foreign body reaction against biodegradable polymer scaffolds affects tissue regeneration during the autologous transplantation of tissue-engineered cartilage in the canine model. Cell Transpl. 21 (7), 1431–1442. 10.3727/096368912x640574 - DOI - PubMed
