Pathogenic variants reveal candidate genes for prostate cancer germline testing for men of African ancestry
- PMID: 41038821
- PMCID: PMC12491615
- DOI: 10.1038/s41467-025-63865-6
Pathogenic variants reveal candidate genes for prostate cancer germline testing for men of African ancestry
Abstract
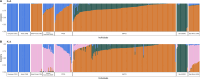
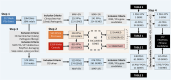
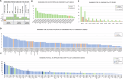
Prostate cancer (PCa) germline testing, while gaining momentum, is ancestry restrictive and African exclusive. Through whole genome sequencing for 217 African ancestral cases (186 southern African, 31 Pan representative), we identify 172 potentially pathogenic variants in 78 DNA damage repair or PCa related genes. Prevalence for reported (13/217, 5.99%) and cumulative predicted (24/217, 11.06%) variants of significance (11 genes) falls below that reported for non-Africans. Conversely, BRCA1, HOXB13, CDK12, MLH1, MSH2, and BRIP1 remain unimpacted. Through pathogenic ranking based on variant frequency and functionality, clinical presentation and tumour-matched biallelic inactivation, top-ranked candidates include PREX2, POLE, FAT1, BRCA2, POLQ, LRP1B and ATM. Besides notable impact of DNA polymerases, including POLG, Fanconi anaemia genes include FANCD2, FANCA, FANCG, ERCC4, FANCE and FANCI, while DNA mismatch repair genes MSH3 and PMS1 outranked known namesakes MSH6 and PMS2. This study provides insights into the spectrum of African-relevant potentially pathogenic PCa variants, highlighting much-needed gene candidates for ancestry-inclusive germline testing.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: Member of Active Surveillance Movember Committee (V.M.H., R.A.E.). Member of external expert committee to Astra Zeneca UK (R.A.E.). Honoraria from GU-ASCO, Janssen, University of Chicago, Dana Farber Cancer Institute USA as a speaker (R.A.E.). Educational honorarium from Bayer and Ipsen (R.A.E.). Member of the SAB of Our Future Health (R.A.E.). Undertakes private practice as a sole trader at The Royal Marsden NHS Foundation Trust and 90 Sloane Street SW1X 9PQ and 280 Kings Road SW3 4NX, London, UK (R.A.E.). The remaining authors declare no competing interests.
Figures
References
-
- National Comprehensive Cancer Network NCCN Clinical Practice Guidelines in Oncology: Prostate Cancer, Version 1.2023 (2023). - PubMed
-
- Giri, V. N. et al. Genetic testing in prostate cancer management: Considerations informing primary care. CA Cancer J. Clin.72, 360–371 (2022). - PubMed
-
- National Comprehensive Cancer Network NCCN Clinical Practice Guidelines in Oncology: Prostate Cancer Early Detection, Version 1.2023 (2023).
MeSH terms
Grants and funding
- C5047/A14835/A22530/A17528, C309/A11566, C368/A6743, A368/A7990, C14303/A17197/Cancer Research UK (CRUK)
- Petre Chair/Petre Foundation
- R01 CA285772/CA/NCI NIH HHS/United States
- 1R01CA285772-01/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
- APP1165762, APP2001098, APP2010551/Department of Health | National Health and Medical Research Council (NHMRC)
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

