Cancer cell dynamics on silica fibers
- PMID: 41038834
- PMCID: PMC12491474
- DOI: 10.1038/s41598-025-16704-z
Cancer cell dynamics on silica fibers
Abstract
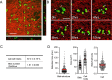
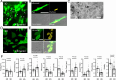
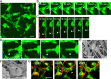
Cancer cells exhibit diverse morphological adaptations in response to the varying environments encountered during cancer progression. However, few experimental platforms enable consistent and comprehensive observation of these dynamic behaviors, particularly during the early phases of cancer cell dissemination through the rigid collagen-rich dermis. In this study, we employed a three-dimensional (3D) nonwoven silica fiber scaffold to investigate how a 3D microenvironment influences cellular membrane dynamics. When fluorescent CT26 cancer cells were seeded onto the silica fiber scaffold, we discovered the cells attached loosely to the fibers displayed stable bleb structures. After initial loose attachment, the cells transitioned to a more anchored state, extending stable, elongated membrane protrusions that were not observed in conventional 2D cultures. Detailed examination of these protrusions further revealed the formation of smaller, perpendicular extensions along the length of the membrane protrusions. The maintenance of elongated membrane structures was regulated by actin dynamics mediated through signaling pathways, including MAP kinase and PI3 kinase. In the spheroid system that mimics primary tumors, Cellbed served as a substrate that accepts detached cancer cells from the spheroids, simulating loose connective tissue containing collagen fibers adjacent to primary tumors. Thus, we found various cancer cell behaviors using the silica fiber scaffold that provides as a valuable platform for reproducibly and comprehensively studying cancer cell dynamics in 3D environments, offering new insights into cell behavior beyond the constraints of 2D culture systems.
Keywords: Cancer; Connective tissues; Fluorescent microscopy; Metastasis; Silica fiber.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

