Predictors of response to neoadjuvant chemo-immunotherapy in metaplastic triple-negative breast cancer
- PMID: 41038871
- PMCID: PMC12491465
- DOI: 10.1038/s41523-025-00816-w
Predictors of response to neoadjuvant chemo-immunotherapy in metaplastic triple-negative breast cancer
Abstract
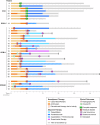
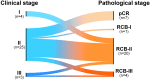
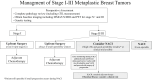
Metaplastic breast cancer (MpBC) treated with standard chemotherapy has low rates of complete pathological response (pCR)(2-23%). In this study, we evaluate the response to neoadjuvant chemo-immunotherapy (NACI) in early-stage MpBC. Thirty-two stage I-III MpBC patients treated with NACI (KEYNOTE-522 regimen) were prospectively enrolled in an institutional rare tumor program. All MpBC were triple negative; most were of chondromyxoid/matrix-producing (12/32, 38%). The majority had stage II (78%) tumors, 12/32 (37.5%) patients completed NACI, 11/32 (34%) progressed during NACI, and in the remaining 9, NACI was discontinued due to side effects. The pCR rate in the entire cohort was 22% (7/32) and it was statistically higher (5/8, 62%) among patients with high ( ≥ 60%) stromal tumor-infiltrating lymphocytes (sTILs) as compared to patients with < 60% sTILs (1/11, 9%). Most patients received adjuvant systemic therapy (capecitabine 16/32, pembrolizumab 20/32). At a median follow-up of 13 months, there were a total of 2 local recurrences, 10 distant recurrences, and 7 deaths. We demonstrated a modest pCR rate in MpBC with the addition of pembrolizumab (22%). Nonetheless, amongst patients with high sTILs, high pCR rates-comparable to those in the KEYNOTE-522 trial-were observed. These findings suggest that sTILs can be used to triage MpBC patients for NACI.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: Nour Abuhadra, abuhadn@mskcc.org: Professional Services and Activities for Boxer Capital, LLC, Medscape, MJH Life Sciences, and OncLive. Fresia Pareja, parejaf@mskcc.org: Membership on the Scientific Advisory Board of MultiplexDx; Consultancy Fees and Membership on the Advisory Board of AstraZeneca; Funding from National Institutes of Health grant No. NIH/NCI P50 CA24779 01 and from a grant from the Starr Cancer Consortium. Stephanie Downs-Canner, downscas@mskcc.org: Funding from National Institutes of Health grant No. NIH/NCI 1 K08 CA259533-01A1. Larry Norton, nortonl@mskcc.org: Founding Editor-in-Chief and Advisory Editor, npj Breast Cancer. Atif Khan, khana7@mskcc.org: Research grants from Merck, Clovis Oncology, and Varian; Patents: Patent awarded for oncology use for the drug riluzole; Stock/Stock Options: Novavax and Xtrava Inc. Sarat Chandarlapaty, chandars@mskcc.org: Editor-in-Chief, npj Breast Cancer; Professional Services and Activities for AstraZeneca, Blueprint Medicines, Casdin Capital, LLC, Daiichi Sankyo, Encore Medical Education. Genesis Therapeutics, Novartis, Prelude Therapeutics, SAGA Diagnostics, and Springer Nature Limited; Uncompensated Professional Services and Activities for Eli Lilly and Company; Equity in Totus Medicines Inc. Pedram Razavi, razavip@mskcc.org: Consultant/Ad Board/Advisor for: Novartis, AstraZeneca, Pfizer, Lilly, Tempus, Prelude Therapeutics, NeoGenomics, Regor Pharmaceuticals, Natera, SAGA Diagnostics, Guardant Health, Myriad, and Foresight Diagnostics; Grant/Research Support: Institutional/grant funding from Grail, Novartis, AstraZeneca, Biothernostics, Tempus, Sophia Genetics, Biovica, Guardant Health, Personalis, Myriad, Foresight Diagnostics, and SAGA Diagnostics. Mark Robson, robsonm@mskcc.org: Professional Services and Activities for Change Healthcare Inc., Clinical Care Options, Genome Quebec, myMedEd, Inc., and WebMD; Uncompensated Professional Services and Activities for Artios Pharma Limited, AstraZeneca, Foundation Medicine, and Pfizer, Inc.All other authors declare no financial or non-financial competing interests.
Figures


References
-
- Tan, P. H. et al. The 2019 World Health Organization classification of tumours of the breast. Histopathology77, 181–185 (2020). - PubMed
-
- Gradishar, W. J. et al. Breast Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc Netw.20, 691–722 (2022). - PubMed
-
- Cortazar, P. et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet384, 164–172 (2014). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

