Preclinical models of melanoma leptomeningeal disease to assess intrathecal checkpoint blockade
- PMID: 41038921
- PMCID: PMC12491558
- DOI: 10.1038/s41598-025-16889-3
Preclinical models of melanoma leptomeningeal disease to assess intrathecal checkpoint blockade
Abstract
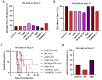
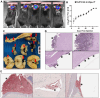
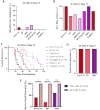
Leptomeningeal disease (LMD) is a subtype of central nervous system metastatic disease that is associated with poor patient outcomes and limited treatment options. There is an unmet need to develop preclinical models of LMD to expedite and improve the development of new therapeutics. Here, we describe the development of multiple orthotopic immunocompetent murine models of melanoma LMD, including their use to assess the efficacy of systemic and/or intrathecal immunotherapy. LMD was established by direct intrathecal injection of murine cell lines (B16-F10, BP, D4M, D4M-UV2, MC38-gp100, RMS, YUMM3.1, and YUMMER1.7) into the cisterna magna of C57BL/6 mice. Tumor take rate, distribution, histology, peri-procedural mortality, and animal survival were assessed for each cell line. Intrathecal and systemic treatment with anti-PD1 were tested for safety, efficacy, and immune infiltration for LMD. Cisternal injection of murine melanoma cell lines successfully established LMD with low peri-procedural mortality, high tumor take rate, and varied survival duration across the panel of cell lines. Decreasing the total number of cells injected and increasing the volume of suspension of the injected cells increased the rate of distal spinal cord deposits, reflecting the common clinical distribution of LMD. Intrathecal administration of anti-PD1 in non-tumor bearing mice caused no morbidity or toxicity. Concurrent intrathecal and systemic anti-PD1 immunotherapy increased the survival of mice with murine melanoma LMD. We have established and characterized several immunocompetent murine models of LMD to facilitate the development and testing of new, more effective immunotherapy strategies for melanoma patients with LMD.
Keywords: Immunotherapy; Intrathecal; Leptomeningeal disease; Melanoma; anti-PD1.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Consent for publication: All authors agree with publication. Ethics approval: The Institutional Animal Care and Use Committee at MD Anderson Cancer Center approved all procedures involving mice in this study (Davies Protocol 00000743-RN02).
Figures



References
-
- Garcia Molina, E. & Penas-Prado, M. Neoplastic meningitis in solid tumours: updated review of diagnosis, prognosis, therapeutic management, and future directions. Neurologia (Engl Ed). 37 (9), 794–805 (2022). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

