Identification and characterization of a putative novel bacteriocin from Bacillus velezensis G02 and its effects on the intestinal microflora in mice
- PMID: 41039210
- PMCID: PMC12492871
- DOI: 10.1186/s12866-025-04357-x
Identification and characterization of a putative novel bacteriocin from Bacillus velezensis G02 and its effects on the intestinal microflora in mice
Abstract
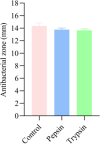
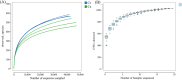
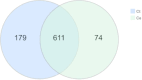
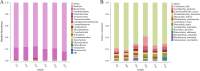
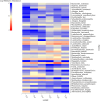
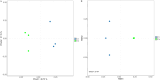
Bacteriocins are defined as proteins that are produced by bacteria and that have antibacterial effects on various pathogenic bacteria. This study explored the effects of the crude extract of bacteriocin produced by Bacillus velezensis G02 (PRJNA1303554) on the intestinal microbiota of normal mice. Observation through liver and kidney tissue sections showed that the bacteriocin crude extract had no obvious adverse reactions on the internal organs of mice, and no mice died. A 16 S rDNA amplicon sequencing analysis revealed that the crude extract of the bacteriocin significantly changed the α-diversity (Shannon (P = 0.51), Simpson (P = 0.51), and Chao1 (P = 0.28)) and β-diversity (PCoA, (P = 0.1) NMDS, (P = 0.0132)) of the intestinal microbiota in normal mice, reducing the species abundance and increasing the evenness of their intestinal microbiota. In addition, the bacteriocin PG02 crude extract exhibited broad-spectrum antibacterial activity against both gram-positive and gram-negative bacteria. The purified bacteriocin was identified by LC-MS/MS in combination with sequence coverage analysis; its molecular weight was found to be 20.1 ~ 31 kDa, and it was speculated to be a putative novel bacteriocin named bacteriocin PG02. This study provides a reference for the microbiology field research and development of feed additive.
Keywords: Bacillus velezensis; Antibacterial; Bacteriocin; Intestinal microbiota.
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The animal study protocol was approved by the Animal Ethics Committee of Guangdong Ocean University, China (IACUC No.: GDOU-LAE-2024-105). and were performed following the Guidelines for Experimental Animals set by the Ministry of Science and Technology (Beijing, China). Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures







References
-
- Frère JM, Rigali S. The alarming increase in antibiotic-resistant bacteria. Drug Target Rev. 2016;3:26–30.
-
- Guarner F, Malagelada JR. Gut flora in health and disease. Lancet. 2003;361(9356):512–9. - PubMed
Grants and funding
- 2023A1515012181/Natural Science Foundation of Guangdong Province
- 2023A1515012181/Natural Science Foundation of Guangdong Province
- 2023A1515012181/Natural Science Foundation of Guangdong Province
- 2023A1515012181/Natural Science Foundation of Guangdong Province
- 2023A1515012181/Natural Science Foundation of Guangdong Province
LinkOut - more resources
Full Text Sources

