IGFBP2 alteration contributes to prostate cancer progression by modulating prostate stroma activation
- PMID: 41039587
- PMCID: PMC12492507
- DOI: 10.1186/s12964-025-02414-6
IGFBP2 alteration contributes to prostate cancer progression by modulating prostate stroma activation
Abstract
Background: Insulin-like growth factor (IGF) binding protein-2 (IGFBP2) is a secretory protein that modulate the activity of IGFs. It is highly expressed in various cancers such as prostate cancer (PCa), in which it may play a controversial role in tumor progression, however, the molecular mechanisms of IGFBP2 in PCa progression remain unclear.
Methods: In this study, we examined the expression pattern and role of IGFBP2 in PCa cells and the stroma during prostate tumor cell progression.
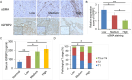
Results: IGFBP2 was highly expressed in LNCaP cells and prostate stromal fibroblasts (PrSC) and was mainly secreted by PrSCs. Tumor cell growth and invasiveness were not directly affected by treatment with IGFBP2 siRNAs (siIGFBP2) or recombinant IGFBP2 (rIGFBP2). However, decreased expression of IGFBP2 significantly increased PrSC activation and the secretion of pro-tumorigenic cytokines IL-6, IL-8, IP10, and CCL5 through upregulation of TGF-β, which subsequently enhanced prostate tumor cell progression. Clinically, low expression of stromal IGFBP2 was associated with a high reactive prostate stroma, advanced PCa progression, and increased IGFBP2 levels in the serum.
Conclusion: Here, we provide mechanistic evidence that IGFBP2 act as a critical regulatory factor in the activation of prostate stromal microenvironment and contributes to aggressive PCa progression.
Keywords: IGFBP2; Insulin-like growth factor binding protein-2; Prostate cancer; Prostate stromal cells.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The Institutional Review Board of the Akita University School of Medicine approved all the experiments, which were performed after obtaining individual written informed consent. Consent for publication: All Authors agreed to the manuscript. Competing interests: The authors declare no competing interests. The authors hereby certify and declare that all authors participated in the study and that all authors have seen and approved the manuscript. I also certify that the contents of this manuscript are not now under consideration for publication elsewhere, and the contents will not be copyrighted, submitted, or published elsewhere while acceptance by the Journal is under consideration.
Figures




References
-
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. - PubMed
-
- Ito K. Prostate cancer in Asian men. Nat Rev Urol. 2014;11:197–212. - PubMed
-
- Kogan-Sakin I, Cohen M, Paland N, Madar S, Solomon H, Molchadsky A, Brosh R, Buganim Y, Goldfinger N, Klocker H, et al. Prostate stromal cells produce CXCL-1, CXCL-2, CXCL-3 and IL-8 in response to epithelia-secreted IL-1. Carcinogenesis. 2009;30:698–705. - PubMed
-
- Ao M, Franco OE, Park D, Raman D, Williams K, Hayward SW. Cross-talk between paracrine-acting cytokine and chemokine pathways promotes malignancy in benign human prostatic epithelium. Cancer Res. 2007;67:4244–53. - PubMed
-
- Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4:505–18. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

