Efficacy and safety of TAS-102 plus Surufatinib in third and later line metastatic pancreatic cancer: a prospective, single center and biomarker exploratory, phase II study
- PMID: 41039600
- PMCID: PMC12492565
- DOI: 10.1186/s12943-025-02437-0
Efficacy and safety of TAS-102 plus Surufatinib in third and later line metastatic pancreatic cancer: a prospective, single center and biomarker exploratory, phase II study
Abstract
Background: Metastatic pancreatic cancer (mPC) has a dismal prognosis, with first line systemic therapy relying primarily on FOLFIRINOX (5FU/irinotecan/oxaliplatin) or AG (Gemcitabine/Nab-Paclitaxel). Therapeutic options for mPC refractory to these regimens remain poorly defined, and data on later-line options are scarce. This prospective, single-arm study evaluated the safety and preliminary efficacy of combining the anti-angiogenic agent surufatinib with the cytotoxic drug TAS-102 (Trifluridine/Tipiracil) in mPC patients who had progressed on ≥ 2 prior lines of therapy.
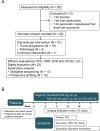
Methods: mPC patients who were refractory to at least 2 previous regimens were enrolled and received TAS-102 (35 mg/m2, po, bid, D1-D5, D8-D12) plus surufatinib (250 mg, po, qd) in a 4-week cycle. The tumor response was assessed by the researcher every 8 weeks and treatment continued until disease progression, unacceptable toxicity, investigator discretion, or patient withdrawal of informed consent. Primary and secondary endpoints included progression-free survival (PFS), overall survival (OS), objective response rate (ORR), disease control rate (DCR), and safety.
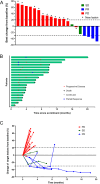
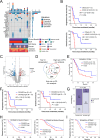
Results: Between January 2023 and June 2024, 22 patients were enrolled into this study. Among 20 patients analyzed for efficacy, median PFS was 2.35 months (95% CI: 1.91-3.94) and median OS was 6.34 months (95% CI: 3.81-10.09). The ORR and DCR were 20% (4/20; all partial response) and 30% (6/20), respectively. All patients experienced treatment emergent adverse events (TEAEs), with anemia (59.1%), neutropenia (54.6%), leukocytopenia (50.0%), and lymphocytopenia (45.5%) as the most common any-grade events. Grade ≥ 3 TEAEs including neutropenia (31.8%), lymphocytopenia (13.6%), and anemia (9.1%), were observed in 50.0% (11/22) of patients. Subgroup analysis identified metastases involving > 2 organs or hepatic sites as potential predicative biomarkers for inferior efficacy. Proteomic screening revealed that overexpressed OCIAD2 correlated with poor prognosis, a finding validated in two publicly available external cohorts (CPTAC database and RuiJin cohort).
Conclusions: Combination of TAS-102 and surufatinib demonstrates clinically meaningful efficacy and manageable toxicity as therapeutic option for later-line mPC. The biomarkers identified in this study may hold the potential to guide patient stratification and warrant further investigation to optimize precision application of this regimen. This study was prospectively registered at clinicaltrials.gov with the number NCT05481463 on August 1st 2022.
Keywords: OCIAD2; Refractory metastatic pancreatic cancer; Surufatinib; TAS-102.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study protocol and related documentation were approved by the Ethics Committee of Sun Yat-sen University Cancer Center and conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice (GCP). Written informed consent was obtained from all enrolled patients. All authors had access to the study data and reviewed and approved the final manuscript. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures



References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. - PubMed
-
- Hu ZI, O’Reilly EM. Therapeutic developments in pancreatic cancer. Nat Rev Gastroenterol Hepatol. 2024;21:7–24. - PubMed
-
- Tempero MA, Malafa MP, Al-Hawary M, Behrman SW, Benson AB, Cardin DB, Chiorean EG, Chung V, Czito B, Del Chiaro M, et al. Pancreatic adenocarcinoma, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19:439–57. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

