Heterogeneity of Extracellular Vesicles and Non-Vesicular Nanoparticles in Glioblastoma
- PMID: 41039818
- PMCID: PMC12491655
- DOI: 10.1002/jev2.70168
Heterogeneity of Extracellular Vesicles and Non-Vesicular Nanoparticles in Glioblastoma
Abstract
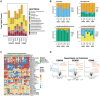
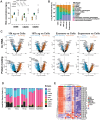
It is increasingly clear that intercellular communication is largely mediated by lipid-bilayer, membrane-bound extracellular vesicles (EVs) and amembranous, non-vesicular extracellular particles (NVEPs), including exomeres and the recently identified supermeres. To elucidate the cargo and functional roles of these carriers, we performed a comprehensive analysis of their lipid, protein and RNA content in the context of colorectal cancer and glioblastoma (GBM). Our results demonstrate that EVs exhibit distinct density profiles correlated with specific biomolecular signatures. Moreover, EVs and NVEPs display notable differences in their protein and RNA composition, which confer distinct functional attributes. Supermeres are notably enriched in components involved in extracellular matrix remodeling and possess the ability to cross the blood-brain barrier, a process dependent on their intact structure and RNA content. Once in the central nervous system (CNS), they preferentially engage with microglia and suppress TGFβ1 expression, suggesting a role in modulating microglial immune activity. Furthermore, systemically administered exogenous supermeres selectively accumulate in GBM tumors in vivo. Together, these findings highlight supermeres as a promising vehicle for delivering therapeutics to the CNS and brain tumors.
Keywords: EGFR; exomeres; extracellular nanoparticles; extracellular vesicles; glioblastoma; supermeres.
© 2025 The Author(s). Journal of Extracellular Vesicles published by Wiley Periodicals LLC on behalf of International Society for Extracellular Vesicles.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Andre, M. , Chambrion C., Charrin S., et al. 2009. “In Situ Chemical Cross‐Linking on Living Cells Reveals CD9P‐1 Cis‐Oligomer at Cell Surface.” Journal of Proteomics 73, no. 1: 93–102. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

