Early results from implementation of HIV pre-exposure prophylaxis (PrEP) with long-acting injectable cabotegravir for people with barriers to oral strategies, Italy, December 2024 to August 2025
- PMID: 41040064
- PMCID: PMC12495377
- DOI: 10.2807/1560-7917.ES.2025.30.39.2500739
Early results from implementation of HIV pre-exposure prophylaxis (PrEP) with long-acting injectable cabotegravir for people with barriers to oral strategies, Italy, December 2024 to August 2025
Abstract
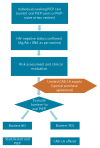
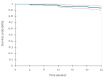
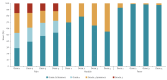
Between December 2024 and August 2025, two Italian HIV/STI clinics offered long-acting injectable cabotegravir (CAB-LA) for HIV prevention in 265 individuals, representing the first European real-world implementation. Participants were prioritised based on vulnerabilities compromising oral pre-exposure prophylaxis (PrEP), such as poor adherence, comorbidities and behavioural barriers. Adherence to injections exceeded 95%, tolerability was favourable and only 1.5% discontinued for drug-related reasons. These findings demonstrate the feasibility of CAB-LA in European clinical services and support its use in specific populations.
Keywords: HIV; PrEP; cabotegravir; implementation; pre-exposure prophylaxis; real-world evidence.
Conflict of interest statement
Figures

References
-
- European Centre for Disease Prevention and Control (ECDC) and WHO Regional Office for Europe. (WHO/Europe). HIV/AIDS surveillance in Europe 2024 – 2023 data. Stockholm and Copenhagen: ECDC and WHO/Europe; 2024. Available from: https://www.ecdc.europa.eu/en/publications-data/hiv-aids-surveillance-eu...
-
- European AIDS Clinical Society (EACS). EACS Guidelines Version 12.1 – Pre-exposure Prophylaxis (PrEP). 2024. Available from: https://eacs.sanfordguide.com/eacs-part1/art/eacs-pre-exposure-prophylaxis
-
- British Association for Sexual Health and HIV (BASHH), & British HIV Association (BHIVA). (2024). Draft BASHH/BHIVA guidelines on the use of HIV pre-exposure prophylaxis (PrEP) (Version 3.0, 24 September 2024). Available from: https://www.bashh.org/_userfiles/pages/files/draft_bashh_bhiva_prep_guid...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
