Bone remodeling stimulated by Wnt-mediated mitophagy regulated extracellular vesicles in subchondral bone contributes to osteoarthritis development
- PMID: 41041044
- PMCID: PMC12486141
- DOI: 10.7150/thno.111724
Bone remodeling stimulated by Wnt-mediated mitophagy regulated extracellular vesicles in subchondral bone contributes to osteoarthritis development
Abstract
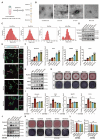
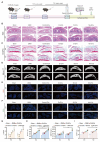
Rationale: Osteoarthritis (OA) is increasingly understood as a disease involving not only cartilage degeneration but also pathological subchondral bone remodeling. The contribution of osteoblast (OB) heterogeneity and their secreted extracellular vesicles (EVs) to this process remains poorly characterized. This study aims to investigate how EVs from distinct OB subtypes modulate subchondral bone remodeling and contribute to OA progression. Methods: OB subtypes representing endothelial (EnOBs), stromal (StOBs), and mineralizing (MinOBs) stages were generated by time-controlled osteogenic induction of BMSCs. EVs were isolated from each OB subtype and characterized by TEM, Western blot, DLS, and miRNA profiling. Functional assays included osteogenic induction, angiogenesis, and cartilage degradation analyses in vitro. RNA-seq and qRT-PCR were used to identify relevant signaling pathways and miRNAs. In vivo effects of EVs were tested in a DMM-induced OA mouse model using intravenous injections, followed by histology, micro-CT, and immunostaining. Results: EVs derived from different OB subtypes exhibited distinct pro-osteogenic, pro-angiogenic, and cartilage-degrading effects. MinOB-derived EVs significantly enhanced osteogenic differentiation and mineralization, correlated with enrichment of calcium phosphate content and specific pro-osteogenic miRNAs. These EVs also carried amorphous calcium phosphate and mitochondrial content, linked to activated mitophagy. Wnt signaling dynamically regulated mitophagy and EV composition, particularly in MinOBs. In vivo, tail vein administration of OB-derived EVs exacerbated subchondral bone sclerosis and cartilage degradation in a time-dependent manner, with MinOB-EVs inducing the most pronounced pathological changes. Conclusions: OB-derived EVs exhibit subtype-dependent regulatory functions in subchondral bone remodeling, mediated by distinct miRNA profiles and mineral cargo shaped by Wnt-regulated mitophagy. These EVs actively participate in OA progression, and their effects vary with disease stage and route of administration. Targeting specific OB subtypes or modulating Wnt-mitophagy signaling may offer novel therapeutic strategies for stage-specific OA intervention.
Keywords: extracellular vesicles; mitophagy; osteoarthritis; osteoblast; subchondral bone.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures





References
-
- Latourte A, Kloppenburg M, Richette P. Emerging pharmaceutical therapies for osteoarthritis. Nat Rev Rheumatol. 2020;16:673–88. - PubMed
-
- Lories RJ, Luyten FP. The bone-cartilage unit in osteoarthritis. Nat Rev Rheumatol. 2011;7:43–9. - PubMed
-
- Karsdal MA, Bay-Jensen AC, Lories RJ, Abramson S, Spector T, Pastoureau P. et al. The coupling of bone and cartilage turnover in osteoarthritis: opportunities for bone antiresorptives and anabolics as potential treatments? Ann Rheum Dis. 2014;73:336–48. - PubMed
-
- Liu J, Wu X, Lu J, Huang G, Dang L, Zhang H. et al. Exosomal transfer of osteoclast-derived miRNAs to chondrocytes contributes to osteoarthritis progression. Nat Aging. 2021;1:368–84. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

