Exploring High-Dose Olanzapine for Psychosis in an Adolescent With Treatment-Resistant Schizophrenia
- PMID: 41041106
- PMCID: PMC12483835
- DOI: 10.7759/cureus.91355
Exploring High-Dose Olanzapine for Psychosis in an Adolescent With Treatment-Resistant Schizophrenia
Abstract
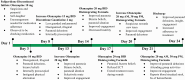
High-dose olanzapine has shown efficacy in treating adult patients with treatment-resistant schizophrenia, though evidence in adolescents remains limited. This report describes the case of a 17-year-old male patient with treatment-resistant schizophrenia who showed significant clinical improvement with a supratherapeutic dose of orally disintegrating olanzapine. The patient, with a 16-month history of schizophrenia and multiple hospitalizations, presented with acute psychosis and aggression after medication non-adherence. Following failed trials of paliperidone and standard-dose olanzapine, and unable to initiate clozapine due to blood draw refusal, the patient was gradually titrated to 50 mg daily of orally disintegrating olanzapine (20 mg early morning, 30 mg at bedtime). This intervention marked improvements in thought process, thought content, and paranoia, with minimal side effects. This case adds to the limited literature on high-dose olanzapine in adolescents. It suggests that supratherapeutic doses may be a viable option for treatment-resistant cases in which standard treatments are ineffective or unfeasible.
Keywords: adolescent psychosis; antipsychotic therapy; clozapine alternatives; high dose olanzapine; orally disintegrating tablets; pediatric schizophrenia; schizophrenia treatment; second generation antipsychotics; supratherapeutic dosing; treatment resistant schizophrenia.
Copyright © 2025, Aggarwal et al.
Conflict of interest statement
Human subjects: Informed consent for treatment and open access publication was obtained or waived by all participants in this study. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures
References
-
- Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Leucht S, Corves C, Arbter D, et al. Lancet. 2009;373:31–41. - PubMed
-
- Umbrella review: association between antipsychotic drugs and metabolic syndrome hallmarks in children and adolescents. Carnovale C, Battini V, Santoro C, et al. J Am Acad Child Adolesc Psychiatry. 2024;63:313–335. - PubMed
-
- Efficacy and safety of aripiprazole for the treatment of schizophrenia: an overview of systematic reviews. Ribeiro EL, de Mendonça Lima T, Vieira ME, Storpirtis S, Aguiar PM. Eur J Clin Pharmacol. 2018;74:1215–1233. - PubMed
Publication types
LinkOut - more resources
Full Text Sources