Medicinal chemistry perspectives on anticancer drug design based on clinical applications (2015-2025)
- PMID: 41041285
- PMCID: PMC12486241
- DOI: 10.1039/d5ra05472a
Medicinal chemistry perspectives on anticancer drug design based on clinical applications (2015-2025)
Abstract
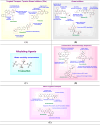
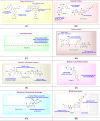
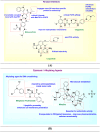
Cancer therapy has undergone a remarkable evolution over the past few decades, driven largely by innovations in medicinal chemistry. This review explores the pivotal role of medicinal chemistry in designing, optimizing, and classifying anticancer agents, from traditional cytotoxics to modern targeted therapies, immunotherapies, and radiotheranostics. The article categorizes FDA-approved anticancer drugs (2015-2025), evaluates their mechanisms of action, structural features, and structure-activity relationships (SAR), and highlights both success stories and challenges in clinical translation. Additionally, it examines withdrawn agents and investigational drugs currently in clinical trials, providing insights into emerging modalities such as PROTACs, antibody-drug conjugates, molecular glues, and AI-driven drug discovery. This synthesis underscores how structure-based drug design, pharmacokinetic modeling, and bioengineering approaches continue to shape the landscape of cancer treatment. Ultimately, medicinal chemistry remains at the heart of the drug development pipeline, offering refined tools for precision oncology and the future of personalized cancer care.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declared no conflict of interest.
Figures

References
-
- Alqaraleha M. Kasabrib V. Muhanac F. Al-Najjarc B. O. Khleifatd K. M. J. Med. Pharm. Chem. Res. 2024;6:1340–1353.
-
- Koper K. Wileński S. Koper A. Phys. Sci. Rev. 2023;8(4):583–604.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

