The role of the tuft cell-interleukin-25 axis in the pathogenesis of inflammatory bowel disease
- PMID: 41041315
- PMCID: PMC12484133
- DOI: 10.3389/fimmu.2025.1629060
The role of the tuft cell-interleukin-25 axis in the pathogenesis of inflammatory bowel disease
Abstract
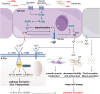
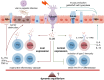
Emerging evidence highlights the tuft cell-Interleukin-25 (IL-25) axis (tuft/IL-25 axis) as a critical orchestrator bridging luminal stimuli and intestinal immunity in Inflammatory Bowel Disease (IBD), which encompasses Crohn's Disease (CD) and Ulcerative Colitis (UC). This review synergises current understanding of how dysregulation within this axis contributes to IBD pathogenesis, arising from disrupted immune homeostasis involving aberrant microbiota responses, genetic susceptibility, and immune pathway dysregulation. Central to this axis, intestinal tuft cells act as chemosensory epithelial sentinels, differentiating in response to microbial and metabolic cues to become the primary source of IL-25. IL-25, signaling via IL-17RB, engages innate and adaptive immune cells, particularly group 2 innate lymphoid cells (ILC2s). While IL-33-responsive homeostatic ILC2s (nILC2s) promote mucosal repair, IL-25-driven inflammatory ILC2s (iILC2s) amplify inflammation, positioning them as pivotal effectors. Critically, IL-25 exhibits a context-dependent "double-edged" role: engagement with IL-25R+ T cells and modulation of downstream signaling can exert anti-inflammatory effects and enhance barrier integrity, yet dysregulation drives pro-inflammatory injury. The axis is dynamically regulated by diverse luminal factors: helminth infection activates the tuft-ILC2 circuit, inducing protective type 2 immunity; specific microbial metabolites (e.g., succinate, SCFAs) modulate its activity; and viral infections can disrupt homeostasis by remodeling tuft cell function. Dysregulation of the tuft/IL-25 axis, driven by infections, microbial metabolite fluctuations, or environmental factors (including regional variations in helminth exposure linked to the hygiene hypothesis), is increasingly recognized as a significant contributor to IBD pathogenesis. Consequently, precisely regulating this axis to harness its beneficial effects while mitigating its detrimental potential represents a promising therapeutic frontier. Future strategies should integrate microbiota remodeling, targeted metabolite interventions, and potentially virus-directed therapies. Furthermore, deeper investigation into the impact of geographical environmental factors on this axis and IBD risk is warranted. Ultimately, multi-pathway approaches aimed at restoring the "immune-microbiota-epithelial" triad via reprogramming the tuft/IL-25 axis hold significant promise for novel IBD management.
Keywords: IL-25; group 2 innate lymphoid cells; inflammatory bowel disease; microbial; tuft cell.
Copyright © 2025 Tao, Li, Zhang, Tang, Zhang, Yang, Jiang, Zhang, Wu and He.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet (London England). (2017) 390:2769–78. doi: 10.1016/S0140-6736(17)32448-0, PMID: - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

