An MRI-derived head-neck finite element model
- PMID: 41042433
- PMCID: PMC12618318
- DOI: 10.1007/s10237-025-02013-x
An MRI-derived head-neck finite element model
Abstract
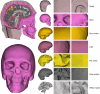
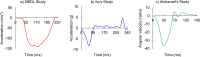
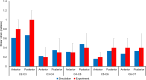
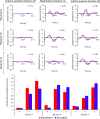
This study aimed to develop and validate a magnetic resonance imaging (MRI)-derived biofidelic head-neck finite element (FE) model comprised of scalp, skull, CSF, brain, dura mater, pia mater, cervical vertebrae, and disks, 14 ligaments, and 42 neck muscles. We developed this model using head and neck MRI images of a healthy male participant and by implementing a novel brain hexahedral meshing algorithm and a scalp erosion model. The model was validated by replicating three experimental studies: Alshareef's brain sonomicrometry study, NBDL's high-acceleration profile, and Ito's frontal impact cervical vertebrae study. The results also showed that the segmented geometries of the model aligned closely with the literature data (within 3 limit). The brain displacement results of the model aligned well (r = 0.48-0.96) with those reported in Alshareef's experimental study. The head-neck kinematic responses of the model showed a strong correlation (r > 0.97) with the NBDL's experimental results. The simulation of Ito's experimental condition yielded peak shear strain values of the cervical spine within 1 of the experimental data. Our developed head-neck FE model provides an effective computational platform for advancing brain and head injury biomechanics research and evaluating protective equipment in various impact scenarios.
Keywords: Computational biomechanics; Finite element method; Image processing; Neck contribution; Simulation and modeling; Traumatic brain injury.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors declare no conflict of interest.
Figures






References
-
- Alshareef A, Giudice JS, Forman J, Salzar RS, Panzer MB (2018) A novel method for quantifying human in situ whole brain deformation under rotational loading using sonomicrometry. J Neurotrauma 35:780–789 - PubMed
-
- Anderst WJ, Donaldson WF III, Lee JY, Kang JD (2015) Three-dimensional intervertebral kinematics in the healthy young adult cervical spine during dynamic functional loading. J Biomech 48:1286–1293 - PubMed
-
- Babiloni F, Babiloni C, Carducci F, Del Gaudio M, Onorati P, Urbano A (1997) A high resolution EEG method based on the correction of the surface Laplacian estimate for the subject’s variable scalp thickness. Electroencephalogr Clin Neurophysiol 103:486–492 - PubMed
-
- Barker JB, Cronin DS (2021) Multilevel validation of a male neck finite element model with active musculature. J Biomech Eng 143:011004 - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

