Cost-Utility Analysis of Accelerated and Standard Strategies for Renal Replacement Therapy Initiation
- PMID: 41042508
- PMCID: PMC12495491
- DOI: 10.1001/jamanetworkopen.2025.35343
Cost-Utility Analysis of Accelerated and Standard Strategies for Renal Replacement Therapy Initiation
Abstract
Importance: Little is known about the long-term costs and outcomes related to strategies for timing of initiation of kidney replacement therapy (KRT) in critically ill patients with severe acute kidney injury (AKI).
Objective: To estimate the cost-utility and cost-effectiveness of accelerated KRT initiation compared with standard KRT initiation in critically ill patients with AKI.
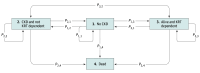
Design, setting, and participants: In this economic evaluation, a state-transition model was developed using data from the Standard vs Accelerated Initiation of Renal Replacement Therapy in AKI (STARRT-AKI) trial, a multicenter, multinational randomized clinical trial of critically ill patients with severe AKI conducted between October 2015 and September 2019. Trial data were linked to administrative health databases in Alberta, Canada, to estimate costs and long-term clinical outcomes. The model included 4 health states: no chronic kidney disease, severe chronic kidney disease, KRT dependent, and dead. Costs are reported in 2024 Canadian dollars. Data were analyzed from February 2022 to November 2024.
Exposure: Initiation of KRT.
Main outcomes and measures: The primary outcome for the economic evaluation was cost per quality-adjusted life-year (QALY) gained. The QALY is a combined measure of patient quality of life and length of life. Expected costs, QALYs, incremental cost-effectiveness ratio (ICER), and incremental net monetary benefit (INMB) were estimated on the basis of 5000 Monte Carlo simulations.
Results: A total of 146 patients from the STARRT-AKI trial were included in the analysis, with 73 patients (mean [SD] age, 59.67 [14.5] years; 52 men [71.3%]) randomized to receive accelerated initiation and 73 patients (mean [SD] age, 61.88 [12.9] years; 48 men [65.8%]) randomized to receive standard initiation. Standard initiation was more costly per patient than accelerated initiation (mean [SD], $251 370 [$155 801] vs $231 518 [$183 302]) but generated more QALYs (mean [SD] 7.49 [2.03] QALYs vs 6.64 [1.76] QALYs). The ICER of standard initiation compared with accelerated initiation was $23 208, with an INMB of $22 648 (95% credible interval, $15 980-$29 316) when assuming a willingness to pay per QALY of $50 000.
Conclusions and relevance: The findings of this economic evaluation suggest that standard KRT initiation may be cost-effective in a Canadian setting, but this finding was sensitive to postdischarge cost trajectories and regional variation in KRT dependence.
Conflict of interest statement
Figures
References
-
- Bagshaw SM, Wald R, Adhikari NKJ, et al. ; STARRT-AKI Investigators; Canadian Critical Care Trials Group; Australian and New Zealand Intensive Care Society Clinical Trials Group; United Kingdom Critical Care Research Group; Canadian Nephrology Trials Network; Irish Critical Care Trials Group . Timing of initiation of renal-replacement therapy in acute kidney injury. N Engl J Med. 2020;383(3):240-251. doi: 10.1056/NEJMoa2000741 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials