Pyrolysis Reactions of (2-Chloroethyl)benzene
- PMID: 41042686
- PMCID: PMC12536402
- DOI: 10.1021/acs.jpca.5c03721
Pyrolysis Reactions of (2-Chloroethyl)benzene
Abstract
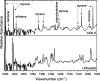
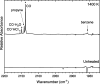
Pyrolysis of polyvinyl chloride (PVC) is considered an alternative to traditional, mechanical methods of recycling. However, there is insufficient research conducted on the thermal decomposition pathways of PVC, particularly the fate of chlorinated hydrocarbons generated during the chemical recycling process. One significant product from the pyrolysis of PVC is (2-chloroethyl)benzene. Using a hyperthermal tubular reactor and matrix-isolation FTIR techniques, the pyrolysis products of gas-phase (2-chloroethyl)benzene were identified. Following pyrolysis at 1400 K, the FTIR spectra indicated the formation of HCl, styrene, phenylacetylene, benzene, vinylacetylene, acetylene, propyne, ethylene, and propargyl radical.
Figures










References
-
- Plastics Europe Plastics – The Fast Facts 2023, https://plasticseurope.org/knowledge-hub/plastics-the-fast-facts-2023/(accessed March 7, 2025).
-
- Edo G. I., Ndudi W., Ali A. B. M., Yousif E., Zainulabdeen K., Onyibe P. N., Ekokotu H. A., Isoje E. F., Igbuku U. A., Essaghah A. E. A.. et al. Poly(vinyl chloride) (PVC): an updated review of its properties, polymerization, modification, recycling, and applications. J. Mater. Sci. 2024;59(47):21605–21648. doi: 10.1007/s10853-024-10471-4. - DOI
-
- Tian Y., Han M., Gu D., Bi Z., Gu N., Hu T., Li G., Zhang N., Lu J.. PVC Dechlorination for Facilitating Plastic Chemical Recycling: A Systematic Literature Review of Technical Advances, Modeling and Assessment. Sustainability. 2024;16(19):8331. doi: 10.3390/su16198331. - DOI
-
- Mnyango J. I., Hlangothi S. P.. Polyvinyl chloride applications along with methods for managing its end-of-life items: A review. Prog. Rubber, Plast. Recycl. Technol. 2024:14777606241308652. doi: 10.1177/14777606241308652. - DOI
LinkOut - more resources
Full Text Sources

