MTCH2 modulates CPT1 activity to regulate lipid metabolism of adipocytes
- PMID: 41044057
- PMCID: PMC12494973
- DOI: 10.1038/s41467-025-63880-7
MTCH2 modulates CPT1 activity to regulate lipid metabolism of adipocytes
Abstract
Metabolic disorders, including obesity and metabolic-associated steatohepatitis, arise from a chronic energy surplus. Thus, enhancing energy dissipation through increased respiration holds significant therapeutic potential for metabolic disorders. Through a comprehensive analysis of human and murine adipose tissues, along with a functional screen, we identify mitochondrial carrier homolog 2, a mitochondrial outer membrane protein, as a pivotal regulator of mitochondrial metabolism. Intriguingly, its expression in adipose tissue is a strong determinant of obesity in humans. Adipocyte-specific ablation of mitochondrial carrier homolog 2 improves mitochondrial function and whole-body energy expenditure, independent of uncoupling protein 1. Furthermore, mitochondrial carrier homolog 2 regulates mitochondrial influx of free fatty acids by modulating the sensitivity of carnitine palmitoyltransferase 1 to malonyl-CoA through direct physical interaction, leading to enhanced energy expenditure in adipocytes/adipose tissue. Here we show mitochondrial carrier homolog 2 functions as a negative regulator of energy metabolism in adipocytes and represents a potential target for treating obesity and related metabolic disorders.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: S.K., B.S.H., A.P., and H.N. are full employees of Boehringer Ingelheim Pharma GmbH & Co. KG, 88397 Biberach an der Riss, Germany. The remaining authors declare no competing interests.
Figures

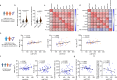
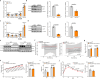

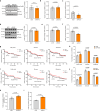

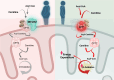
References
-
- GBD 2013 Risk Factors Collaborators et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet386, 2287–2323 (2015). - PMC - PubMed
-
- Cannon, B. & Nedergaard, J. Cell biology: neither brown nor white. Nature488, 286–287 (2012). - PubMed
-
- Bódis, K. & Roden, M. Energy metabolism of white adipose tissue and insulin resistance in humans. Eur. J. Clin. Investig.48, e13017 (2018). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

