Comparison of major, minor and junctional circumsporozoite protein epitopes for malaria vaccine design
- PMID: 41044073
- PMCID: PMC12494720
- DOI: 10.1038/s41541-025-01264-0
Comparison of major, minor and junctional circumsporozoite protein epitopes for malaria vaccine design
Abstract
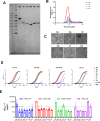
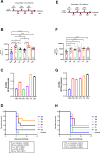
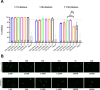
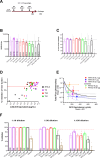
Currently approved malaria vaccines (RTS,S/AS01 and R21/Matrix-M) contain the tetrapeptide major repeats (19x NPNA) and C-terminal domain of the circumsporozoite protein of Plasmodium falciparum. Incorporating the junctional (NPDP) and minor repeat (NPNV) epitope targeted by protective human monoclonal antibodies into immunogens is hypothesized to improve vaccine efficacy. However, comparisons of such candidates have yielded contradictory results due to inter-study differences. Tobacco mosaic virus (TMV) capsid virus-like particles displaying the minor repeat, junctional, and major repeat epitopes were compared in an intravenous challenge model. Despite high cross-reactivity and in vitro inhibition, minor repeat candidates did not confer sterile protection in vivo. Constructs displaying major repeats NPNAx20, NPNAx5, and a junctional+minor repeat epitope induced sterile protection. Head-to-head comparisons of selected TMV vaccines and RTS,S revealed equivalent in vivo liver burden reduction. TMV-NPNAx20 was selected for clinical-grade antigen manufacture based on its equivalent reduction in parasite burden at lower antibody concentrations.
© 2025. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Competing interests: F.Z. serves as an editor of this journal and had no role in the peer review or decision to publish this manuscript. All other authors declare no competing interests.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

