All-optical voltage interrogation for probing synaptic plasticity in vivo
- PMID: 41044179
- PMCID: PMC12494759
- DOI: 10.1038/s41467-025-63867-4
All-optical voltage interrogation for probing synaptic plasticity in vivo
Abstract
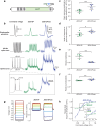
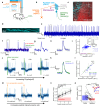
Measuring synaptic efficacy and defining the rules for induction of synaptic plasticity at identified connections in the mammalian brain is essential for understanding how synapses contribute to learning and memory. This requires new approaches to selectively evoke presynaptic activity and measure postsynaptic responses with high spatiotemporal resolution and high sensitivity over long periods in vivo. Here we develop an all-optical approach to probe synaptic plasticity at identified cerebellar synapses in awake, behaving mice. We developed and applied JEDI-2Psub, a genetically encoded voltage indicator with increased sensitivity around resting membrane potentials, to record subthreshold and suprathreshold activity in Purkinje cell (PC) dendrites while selectively activating their granule cell (GrC) inputs using optogenetics and their climbing fiber (CF) inputs using sensory stimulation. We measured synaptic potentials and complex spike signals across the dendrites of multiple neighboring PCs, enabling us to examine correlations in voltage signals within and between neurons. We show how pairing GrC activity with sensory-evoked CF inputs can trigger long-term plasticity of inhibitory responses in PCs. These results provide a blueprint for defining the rules for plasticity induction at identified synapses in awake animals during behavior.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: F.S.-P. holds a US patent for a voltage sensor design (patent #US9606100 B2) that encompasses the GEVI reported here. The other authors declare no competing interests.
Figures


References
-
- Luo, L. Principles of Neurobiology 2nd edn (CRC Press/Garland Science, 2020).
-
- Markram, H., Lübke, J., Frotscher, M. & Sakmann, B. Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science275, 213–215 (1997). - PubMed
-
- Meliza, C. D. & Dan, Y. Receptive-field modification in rat visual cortex induced by paired visual stimulation and single-cell spiking. Neuron49, 183–189 (2006). - PubMed
MeSH terms
Grants and funding
- R61 CA278458/CA/NCI NIH HHS/United States
- 224688/WT_/Wellcome Trust/United Kingdom
- R01 NS146078/NS/NINDS NIH HHS/United States
- MR/T022922/1/RCUK | Medical Research Council (MRC)
- R01 NS136027/NS/NINDS NIH HHS/United States
- AdG 695709/EC | EC Seventh Framework Programm | FP7 Ideas: European Research Council (FP7-IDEAS-ERC - Specific Programme: "Ideas" Implementing the Seventh Framework Programme of the European Community for Research, Technological Development and Demonstration Activities (2007 to 2013))
- RF1 NS128901/NS/NINDS NIH HHS/United States
- R01 NS146023/NS/NINDS NIH HHS/United States
- R01 EB032854/EB/NIBIB NIH HHS/United States
- 201225/WT_/Wellcome Trust/United Kingdom
- U01 NS133971/NS/NINDS NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- P50 HD103555/HD/NICHD NIH HHS/United States
- BB/N009835/1/RCUK | Biotechnology and Biological Sciences Research Council (BBSRC)
LinkOut - more resources
Full Text Sources
Research Materials

