Evaluating Parkinson's disease biomarkers in substantia nigra following sublethal γ-radiation exposure in a large animal model
- PMID: 41044307
- PMCID: PMC12494885
- DOI: 10.1038/s41531-025-01136-3
Evaluating Parkinson's disease biomarkers in substantia nigra following sublethal γ-radiation exposure in a large animal model
Abstract
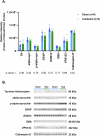
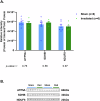
Idiopathic Parkinson's Disease (iPD) involves genetic and environmental factors, including ionizing radiation. While high-dose radiation induces neurodegeneration, the effects of low-dose radiation (LDR) remain unclear. This study examined the impact of a single acute total-body LDR exposure (1.79 Gy) on the substantia nigra (SN) of swine, a large mammal model closely resembling humans. Fourteen male Göttingen minipigs were assigned to radiation (RAD; n = 6) or sham (SH; n = 8) groups. We analyzed iPD-related markers (α-synuclein, phosphorylated α-syn, tyrosine hydroxylase), genetic PD markers (LRRK2, GBA, VPS13C, Cathepsin D), neuroinflammation (GFAP), and mitochondrial proteins (ATP5A, SDHB, NDUF8). No significant molecular, histological, or immunohistochemical differences were observed between RAD and SH animals. LRRK2 was undetectable, and no structural damage or neuroglial changes were found. These findings suggest that single acute LDR exposure does not elicit short-term PD-related alterations in the SN of swine, although long-term or cumulative effects warrant further investigation.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

References
-
- Pringsheim, T., Jette, N., Frolkis, A. & Steeves, T. D. The prevalence of Parkinson’s disease: a systematic review and meta-analysis. Mov. Disord.29, 1583–1590 (2014). - PubMed
Grants and funding
- 312516-1.00-66721/DoD/USU Brain Tissue Repository and Neuropathology Research Program
- 312516-1.00-66721/DoD/USU Brain Tissue Repository and Neuropathology Research Program
- 312516-1.00-66721/DoD/USU Brain Tissue Repository and Neuropathology Research Program
- DM178018/US Defense Medical Research and Development Program
LinkOut - more resources
Full Text Sources
Miscellaneous

