Development of cognitive, motor, metabolic, and mutant huntingtin aggregation in the zQ175 mouse model of Huntington's disease
- PMID: 41044411
- PMCID: PMC12494737
- DOI: 10.1038/s41598-025-17956-5
Development of cognitive, motor, metabolic, and mutant huntingtin aggregation in the zQ175 mouse model of Huntington's disease
Abstract
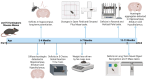
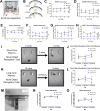
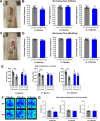
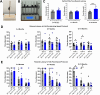
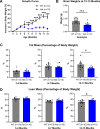
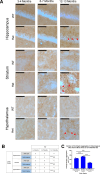
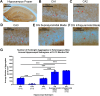
Huntington's disease (HD) is an inherited neurodegenerative disease. In humans, the clinical diagnosis is often dependent on the emergence of motor symptoms. However, cognitive impairments and metabolic changes can be early indicators. HD mouse models are a useful tool to understand disease progression, however, relatively few studies have monitored the timeline for the emergence of cognitive indices with motor and metabolic phenotypes in parallel. In this study, cognitive, motor, and metabolic phenotypes were investigated at different ages in the zQ175 knock-in mouse alongside immunohistochemical and long-term potentiation (LTP) studies. We demonstrated that zQ175 mice developed impaired hippocampal LTP at 3-months and cognitive deficits in visuospatial attention were evident by 4-months. Long-term and spatial memory impairments emerged by 12-months, alongside motor impairments. Additionally, an anxiolytic-like phenotype emerged at 6-months. Differences in body weight were also detected from 6-months onwards, primarily driven by a reduction in fat mass. Additionally, reduced brain weight and the presence of huntingtin aggregates in the hippocampus, striatum and hypothalamus were observed at 12-months. These data support the zQ175 mouse as a model of HD, which recapitulates many aspects of the disease progression in humans and can be used to understand mechanisms underlying the disease.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

References
-
- MacDonald, M. E. et al. A novel gene containing a trinucleotide repeat that is expanded and unstable on huntington’s disease chromosomes. Cell72 (6), 971–983 (1993). - PubMed
-
- Duyao, M. et al. Trinucleotide repeat length instability and age of onset in huntington’s disease. Nat. Genet.4 (4), 387–392 (1993). - PubMed
-
- Andrew, S. E. et al. The relationship between trinucleotide (CAG) repeat length and clinical features of huntington’s disease. Nat. Genet.4 (4), 398–403 (1993). - PubMed
-
- Novak, M. J. & Tabrizi, S. J. Huntington’s disease. Bmj. 340, 30 (2010). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

