Multi-omics analyses reveal the defense mechanisms behind the tolerance of the 'Parson Brown' sweet orange to Huanglongbing
- PMID: 41044627
- PMCID: PMC12495878
- DOI: 10.1186/s12870-025-07372-2
Multi-omics analyses reveal the defense mechanisms behind the tolerance of the 'Parson Brown' sweet orange to Huanglongbing
Abstract
Background: 'Parson Brown' sweet orange is an early-maturing variety and is considered a resilient tree in the face of Huanglongbing (Citrus Greening) disease. Its ability to maintain productivity under endemic HLB conditions has demonstrated its value for growers battling this devastating disease. This study compared the metabolomic profile, transcriptomic analysis, and physiological responses of three early-maturing sweet oranges: 'Hamlin', 'Roble', and 'Parson Brown'.
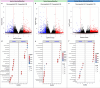
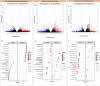
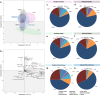
Results: Healthy greenhouse-grown trees were propagated and exposed to 'Candidatus Liberibacter asiaticus' via psyllid infestation. We recorded a decrease of landed psyllids on 'Parson Brown' (20.58%) compared to 'Hamlin' (34.38%) and 'Roble' (45.04%), in addition to a lower 'Ca. L. asiaticus' titer in 'Parson Brown'. Transcriptomic profiling indicated cultivar-specific expression patterns, with 'Parson Brown' showing strong upregulation of genes involved in terpenoid and flavonoid biosynthesis. Infected 'Parson Brown' trees exhibited significantly higher total phenolic and flavonoid contents, lower ROS and H₂O₂ levels, and enhanced expression of antioxidant-related genes. Volatile analysis revealed distinct profiles in 'Parson Brown', including elevated levels of certain monoterpenes, which may contribute to reduced vector attraction.
Conclusion: The tolerance of 'Parson Brown' is driven by a multifaceted defense response, emphasizing the value of traditional breeding in combining diverse resistance traits from parental lines.
Keywords: Citrus greening; Disease tolerance; Huanglongbing; Metabolomics; Sweet orange; Terpene biosynthesis; Transcriptomics.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures





References
-
- Bové JM. Huanglongbing: a destructive, newly-emerging, century-old disease of citrus. J Plant Pathol. 2006;88:7–37.
-
- Wang N, Trivedi P. Citrus huanglongbing: a newly relevant disease presents unprecedented challenges. Phytopathology. 2013;103(7):652–65. - PubMed
-
- Stover E, McCollum GT, Driggers R, Lee R, Shatters R Jr, Duan Y, Ritenour M, Chaparro JX, Hall DG. Resistance and tolerance to Huanglongbing in citrus. Acta Hortic. 2015;1065:899–903.
-
- USDA. Florida Orange Production Plummeted for Years. Citrus Industry. 2024.
MeSH terms
LinkOut - more resources
Full Text Sources

