Bridging Implementation Gaps in Digital Health: A Translational Research Imperative for Equitable Healthcare Innovation
- PMID: 41044865
- PMCID: PMC12494540
- DOI: 10.1111/cts.70375
Bridging Implementation Gaps in Digital Health: A Translational Research Imperative for Equitable Healthcare Innovation
Abstract
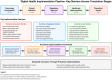
Digital health technologies' potential to democratize healthcare access appears constrained by implementation challenges that may reproduce existing inequities. This paper examines systemic barriers potentially impeding translation, suggesting prevailing frameworks inadequately address structural determinants. Despite substantial investment, limited FDA approvals for digital-derived endpoints indicate possible regulatory-innovation disjunctures, while constrained funding and incomplete reimbursement structures systematically exclude essential services. We propose a precision implementation framework repositioning implementation science as integral to technological development, potentially challenging linear translational paradigms. This approach emphasizes examining sociotechnical systems, organizational readiness variations, and community-specific contexts. Whether digital health mitigates or exacerbates disparities likely depends on reconceptualizing implementation as socio-organizational transformation requiring regulatory harmonization, sustainable economic models, and equity-centered engagement.
Keywords: digital health; health equity; implementation science; precision implementation; translational research.
© 2025 The Author(s). Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Zullig L. L., Blue C., Kelley M. J., et al., “The Veterans Affairs Health Care System National Teleoncology Service: Veteran and Provider Perspectives and Experiences,” Cancer Survivorship Research & Care 2, no. 1 (2024): 2329363.
-
- Digital Medicine Society , “V3+ Framework: Extending Verification, Analytical Validation, and Clinical Validation to Include Usability Validation,” 2024, https://dimesociety.org/access‐resources/extending‐v3/.
-
- Food and Drug Administration , “Digital Health Technologies for Remote Data Acquisition in Clinical Investigations: Guidance for Industry, Investigators, and Other Stakeholders. Final Guidance,” 2023.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical