Development and validation of a prediction score for early identification of palliative care needs for patients in the intensive care unit: a multicentre retrospective cohort study
- PMID: 41048666
- PMCID: PMC12495436
- DOI: 10.1016/j.eclinm.2025.103519
Development and validation of a prediction score for early identification of palliative care needs for patients in the intensive care unit: a multicentre retrospective cohort study
Abstract
Background: Many critically ill patients and their families face serious physical, psychosocial, and spiritual burdens which can be addressed through specialist palliative care (PC). Identifying PC need and patients who would benefit from PC consultation remains challenging. We aimed to develop and validate a simple and accurate score for predicting PC involvement during intensive care unit (ICU) treatment with predictors routinely collected within 24 h of ICU admission to enable early integration.
Methods: This multicentre retrospective cohort study included adult patients admitted to an ICU between Jan 01, 2011 and Dec 31, 2022 in Boston, MA (development cohort), Omaha, NE, and Atlanta, GA (validation cohort [VC] I and II, respectively) in the United States of America. For score development, cases with missing data were excluded. PC involvement was defined as a specialist PC consult request or note in the patient's medical record. Candidate predictors were selected using adaptive-lasso-logistic regression-models with 10-fold cross-validation. We developed a comprehensive epidemiological score and subsequently a simplified version (PC-ICU) for clinical use. Score performance was quantified using the area-under the receiver-operating-characteristic (AU-ROC) curve and the PC-ICU score was externally validated in two independent cohorts. The PC-ICU score is available at: www.pc-icu.com.
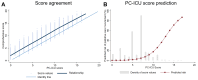
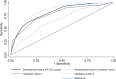
Findings: 99,582 patients (55.1% male, 44.9% female) were included. Score development was performed in 60,091 patients (19,705/79,796 excluded due to missing data), of which 5.5% (n = 3294) received PC (VC I: 1449 included patients [9.7%, n = 141 received PC], VC II: 38,042 included patients [13.2%, n = 5038 received PC]). The PC-ICU score consists of ten factors, including demographics, comorbidities, admission data, and the current patient status. AU-ROC curves in the development cohort were 0.85 (95% CI 0.84-0.86) and 0.81 (95% CI 0.81-0.82] for the comprehensive and PC-ICU score, respectively, indicating excellent discriminative ability. External validation of the PC-ICU score indicated good accuracy (AU-ROC of 0.78 [95% CI 0.74-0.82, VC I]; 0.67 [95% CI 0.66-0.67, VC II]).
Interpretation: The PC-ICU score predicts PC consultation during intensive care upon ICU admission. This externally validated instrument can identify patients with potential PC needs and may facilitate integration of multi-disciplinary care. Additional prospective evaluation of the PC-ICU score in different national and international locations, healthcare settings and patient populations is needed to further enhance generalisability.
Funding: The Deutsche Forschungsgemeinschaft (German Research Foundation).
Keywords: Critical care; End-of-life care; Interdisciplinary care; Palliative care; Quality of life.
© 2025 The Author(s).
Conflict of interest statement
TT was funded by the German Research Foundation to conduct this work (Walter Benjamin Fellowship, project number: 522518834). TT received travel support to attend conferences through a DAAD (German Academic Exchange Service, Deutscher Akademischer Austauschdienst) congress stipend and a HeRa (Heine Research Academies) congress travel grant. EA is an associate editor for BMC Anesthesiology. LJW is an associate editor for BMC Anesthesiology and received funding for an investigator-initiated study from Merck & Co., which does not pertain to this manuscript. MSS received an unrestricted philanthropic grant from Jeffrey and Judith Buzen. MSS is an associate editor for BMC Anesthesiology. He received honoraria for lectures from Fisher & Paykel Healthcare and Mindray Medical International Limited. MSS received funding for investigator-initiated studies from Merck & Co., which do not pertain to this manuscript. All funders had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. RH, EM, SRe, SRi, BSP, CG, SK, JS, MS, RMA, KL and MN declare no competing interests.
Figures

References
LinkOut - more resources
Full Text Sources
Research Materials

