Exploring Regioisomeric Indole-Furanone Tubulin Inhibitors
- PMID: 41048723
- PMCID: PMC12489669
- DOI: 10.1021/acsomega.5c07360
Exploring Regioisomeric Indole-Furanone Tubulin Inhibitors
Abstract
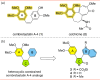
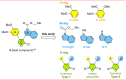
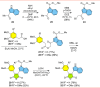
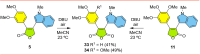
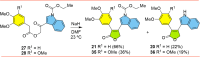
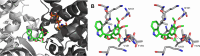
Tubulin is involved in microtubule function and affects mitosis, cell shape, migration, and the movement of organelles. Consequently, tubulin inhibitors have emerged as promising targets for cancer treatment. We previously identified a novel antitubulin motif that combines a furanone, indole, and electron-rich dimethoxyphenyl ring. The lead indole-furanone compound (3) demonstrated submicromolar potency on cancer cells and inhibited tubulin polymerization. To advance these findings, we synthesized a small library of analogs of 3, analyzed their biological activities, and used molecular modeling to elucidate binding interactions in the tubulin colchicine binding site. To assess the impact on potency, we compared: (1) dimethoxy vs trimethoxy substitution of the phenyl A-ring, (2) N-indole substitution of the indole B-ring, and (3) regioisomers and anhydrides of the furanone C-ring. In the process of developing the synthesis of the furanone C-ring regioisomers, we identified that a modification of conditions (NaH/inert vs DBU/air) could be used to give either the corresponding furanones or maleic anhydrides. Of the 18 synthesized compounds, six are biologically active with two exhibiting submicromolar activity against HL-60 cells. Of the six active compounds, (1) three contained dimethoxyphenyl A-rings and three contained trimethoxyphenyl A-rings largely oriented toward the tubulin α-subunit, (2) the N-indole substitution appeared to be less impactful on activity although having the indole nitrogen pointing down into the colchicine binding site was favored, and (3) the furanone carbonyl group located cis to the di- or trimethoxyphenyl A-ring and pointing toward the α-subunit was favored.
© 2025 The Authors. Published by American Chemical Society.
Figures



References
-
- Podolak M., Holota S., Deyak Y., Dziduch K., Dudchak R., Wujec M., Bielawski K., Lesyk R., Bielawska A.. Tubulin inhibitors. Selected scaffolds and main trends in the design of novel anticancer and antiparasitic agents. Bioorg. Chem. 2024;143:107076. doi: 10.1016/j.bioorg.2023.107076. - DOI - PubMed
LinkOut - more resources
Full Text Sources
