Combined Esketamine and Dexmedetomidine Decreases the Risk of Postoperative Delirium in Neurosurgical Pediatrics: A Randomized Controlled Trial
- PMID: 41049041
- PMCID: PMC12495960
- DOI: 10.2147/DDDT.S550647
Combined Esketamine and Dexmedetomidine Decreases the Risk of Postoperative Delirium in Neurosurgical Pediatrics: A Randomized Controlled Trial
Abstract
Purpose: Present study was designed to investigate whether combined esketamine and dexmedetomidine could decrease the risk of postoperative delirium in neurosurgical pediatric.
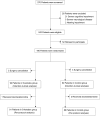
Patients and methods: In this prospective randomized controlled study, pediatrics (aged 2-16 years) who were scheduled for selective neurosurgery were enrolled. Patients were randomized to receive combined esketamine (0.5 mg/kg) and dexmedetomidine (0.5 μg/kg) or normal saline as placebo at anesthesia induction. Primary outcome was the incidence of delirium within postoperative the first five days which was assessed twice daily using the Cornell Assessment of Pediatric Delirium (CAPD). CAPD≥ 10 at any assessment point was considered as delirium. Secondary outcomes included the incidence of emergence delirium, non-delirium complications within postoperative 30 days, postoperative length of in-hospital stay, and medical cost during hospitalization. The change of systematic inflammation was reflected by Neutrophil-to-Lymphocyte Ratio.
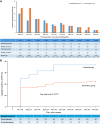
Results: From July 2021 to March 2023, 270 patients were screened, and 190 patients were randomized. Median age of enrolled patients was similar between two groups (63.0 [43.3, 111.3] vs 70.0 [46.3, 114.8] months, P = 0.883). Patients in esketamine-dex group suffered lower incidence of postoperative delirium than control group (intention-to-treat analysis: 22.8% [21/92] vs 38.0% [35/92], RR = 0.600, 95% CI 0.380-0.948, P = 0.025; per-protocol analysis: 22.0% [20/91] vs 38.2% [34/89], RR = 0.575, 95% CI 0.360-0.919, P = 0.018). The incidence of emergence delirium was lower in esketamine-dex group than control group (20.7% [19/92] vs 50.0% [46/92], RR = 0.413, 95% CI 0.263-0.648, P < 0.001). Patients in esketamine-dex group had lower medical cost (P < 0.001), and there was no statistical significance in the incidence of non-delirium complications within postoperative 30 days and postoperative length of in-hospital stay. The neutrophil-to-lymphocyte ratios at postoperative first and third day were comparable between two groups. Safety outcomes such as bradycardia and hypotension were comparable between groups.
Conclusion: Combined esketamine and dexmedetomidine could decrease the risk postoperative delirium and emergence delirium in pediatrics after neurosurgery.
Keywords: delirium; dexmedetomidine; esketamine; neurosurgery; pediatric.
© 2025 Wen et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

