Trapping DNA Radicals With DMPO Reduces Hypochlorous Acid-Induced 8-oxo-7,8-dihydro-2'-deoxyguanosine and Mutagenesis in Lung Epithelial Cells
- PMID: 41049128
- PMCID: PMC12494474
- DOI: 10.1155/omcl/8868348
Trapping DNA Radicals With DMPO Reduces Hypochlorous Acid-Induced 8-oxo-7,8-dihydro-2'-deoxyguanosine and Mutagenesis in Lung Epithelial Cells
Abstract
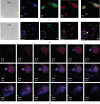
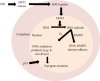
Pulmonary neutrophilic inflammation (PNI) is the recruitment and activation of neutrophils in the microvasculature with the release of myeloperoxidase (MPO) in the airways. Bystander epithelial cells can take up MPO, where it can generate HOCl. HOCl can react with DNA, generating DNA radicals, which then decay to produce several mutagenic end-oxidation products, such as 8-oxo-7,8-dihydro-2'-deoxyguanosine (8-oxo-dGuo). Herein, we aimed to test whether HOCl-induced DNA radicals precede DNA oxidation and mutagenesis in A549 human lung epithelial cells as an in vitro model that resembles PNI. Interestingly, by trapping HOCl-induced DNA radicals, the nitrone spin trap 5,5-dimethyl-1-pyrroline N-oxide (DMPO) blocks the formation of 8-oxo-dGuo and possibly other end-oxidation products, forming DNA-DMPO nitrone adducts. By preventing DNA oxidation, DMPO reduces the mutation of the hypoxanthine phosphoribosyl transferase (hrpt) gene, one of the genes most sensitive to oxidative damage. The transcription factor p53 is known as the master regulator of the cell response to genomic damage. By trapping DNA radicals, DMPO also blocks the translocation of p53 to the cell nucleus, suggesting that by trapping DNA radicals with DMPO, end-oxidation products are prevented, and the cell response to genomic damage is blunted. Trapping DNA radicals to reduce the accumulation of HOCl-induced mutagenic end-oxidation products will provide new therapeutic avenues to reduce genotoxic damage during PNI.
Keywords: 8-oxo-dGuo; DNA radical; DNA-DMPO nitrone adduct; cell model; hrpt gene mutation; pulmonary neutrophilic inflammation.
Copyright © 2025 C. M. Lopez et al. Oxidative Medicine and Cellular Longevity published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Mejiba S. E. G., Ramirez D. C. Neutrophilic Inflammation and Diseases: Pathophysiology, Biomarkers and Therapeutic Targets . Republica de Moldova ELIVA Press S.R.L; 2020.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

