Exosomes in arteriovenous fistula stenosis
- PMID: 41049292
- PMCID: PMC12492956
- DOI: 10.3389/fcell.2025.1663973
Exosomes in arteriovenous fistula stenosis
Abstract
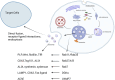
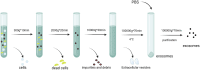
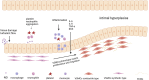
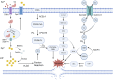
Arteriovenous fistula (AVF) stenosis is a complex pathological process caused by venous intimal hyperplasia, and its development is influenced by factors such as surgical injury, hemodynamic changes, inflammatory responses, and cellular proliferation and migration. Exosomes are critical mediators of intercellular communication and carry biomolecules (e.g., deoxyribonucleic acid, ribonucleic acid [RNA], and proteins) that can regulate cell functions and impact inflammatory responses, endothelial cell proliferation, and vascular smooth muscle cell migration. Studies have shown that molecules such as microRNAs within exosomes play significant roles in vascular stenosis-related diseases and can function as potential therapeutic tools and biomarkers for disease diagnosis. In addition, exosomes can serve as drug carriers with good biocompatibility and targeting capabilities, providing new avenues for the diagnosis and treatment of AVF stenosis. This article reviews the application of exosomes in AVF stenosis.
Keywords: arteriovenous fistula (AVF); chronic kidney disease (CKD); endothelial-to-mesenchymal transition (EndoMT); exosomes; extracellular vesicles; intimal hyperplasia; vascular remodeling.
Copyright © 2025 Cao, Guo, Chen, Li, Jie and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources

