Targeting LC3B/MCL-1 expression by Protodioscin induces autophagy and apoptosis in human glioblastoma cells in vitro and in vivo
- PMID: 41049425
- PMCID: PMC12492353
- DOI: 10.7150/ijms.116656
Targeting LC3B/MCL-1 expression by Protodioscin induces autophagy and apoptosis in human glioblastoma cells in vitro and in vivo
Abstract
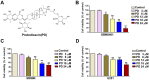
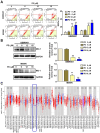
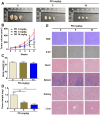
Protodioscin (PD), a natural steroidal saponin extracted from Dioscorea and Tribulus terrestris, has been shown to exhibit anticancer, antimetastatic, and pro-autophagic and apoptotic activities in various malignant tumor cells. However, its antitumor potential and molecular mechanisms in glioblastoma remain unclear. This study aimed to investigate the effects of PD on apoptosis and autophagy in human glioblastoma cells using both in vitro and in vivo models. Our results suggested that PD significantly inhibited the proliferation, induced mitochondrial dysfunction and apoptosis of human GBM8401 and M059K cells, as evidenced by the activation of cleaved-PARP (c-PARP) and MCL-1 expression. However, PD also enhanced autophagic activity, as indicated by the upregulation of LC3B expression. Silencing LC3 expression using siRNA markedly attenuated PD-induced autophagy and apoptosis, these results demonstrated the crucial regulatory role of LC3 in mediating cell death pathways. In an in vivo GBM8401 xenograft model, PD treatment significantly suppressed GBM8401 tumor growth without affecting body weight or causing organ toxicity in PD-treated mice. Immunohistochemical analysis further suggested that PD reduced the expression of the Ki67 expression in glioblastoma tumor tissues and molecular docking finding that PD strong interaction with LC3B and MCL-1. In summary, PD induces both apoptosis and autophagy in glioblastoma cells through LC3B/MCL-1 modulation, demonstrating strong antitumor potential against human glioblastoma. These findings suggest that PD may serve as a novel therapeutic strategy and a promising candidate for glioblastoma treatment.
Keywords: Apoptosis; Autophagy; Glioblastoma; LC3; MCL-1; Protodioscin.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



References
-
- Tagde P, Tagde P, Tagde S, Bhattacharya T, Garg V, Akter R. et al. Natural bioactive molecules: An alternative approach to the treatment and control of glioblastoma multiforme. Biomed Pharmacother. 2021;141:111928. - PubMed
-
- Navarro Del Hierro J, Casado-Hidalgo G, Reglero G, Martin D. The hydrolysis of saponin-rich extracts from fenugreek and quinoa improves their pancreatic lipase inhibitory activity and hypocholesterolemic effect. Food Chem. 2021;338:128113. - PubMed
-
- He X, Qiao A, Wang X, Liu B, Jiang M, Su L. et al. Structural identification of methyl protodioscin metabolites in rats' urine and their antiproliferative activities against human tumor cell lines. Steroids. 2006;71:828–33. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

