mir-31 mediated control of bacteriome size in tsetse flies
- PMID: 41049555
- PMCID: PMC12495243
- DOI: 10.1016/j.cris.2025.100117
mir-31 mediated control of bacteriome size in tsetse flies
Abstract
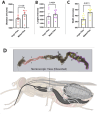
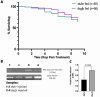
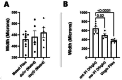
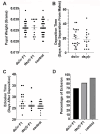
Tsetse flies are the primary vectors of African trypanosomes, which are transmitted through blood feeding. To supplement this nutritionally limited diet, tsetse evolved an obligate mutualism with the bacterium Wigglesworthia glossinidia, housed within a specialized organ called the bacteriome. While the functional contributions of this symbiosis towards tsetse fitness have been studied, host morphological changes that accommodate this relationship remain less understood. In pregnant flies, variable expression of microRNAs (miRNAs) regulates protein expression, but the specific impacts are unknown. During pregnancy, high expression of fatty acyl-CoA reductase (far) within the bacteriome is indirectly correlated with miR-31 abundance and coincides with bacteriome size increase. We explored the roles of far and miR-31 towards this morphological change. Although RNAi effectively reduced far expression, bacteriome size still increased, suggesting its expansion is independent of far. In contrast, disrupting miR-31 activity resulted in significantly enlarged bacteriomes in virgin flies, resembling those of mated females. These results suggest that gene(s) other than far are regulated by miR-31 and may contribute to bacteriome remodeling during pregnancy, potentially to meet increased symbiosis demands. Ultimately, disrupting this obligate mutualism may present a promising target for future vector control strategies.
Keywords: Bacteriome; Glossina; MicroRNA; Symbiosis; tsetse.
© 2025 The Authors. Published by Elsevier B.V.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Rita V.M. Rio reports financial support was provided by West Virginia University. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Abro Z., Kassie M., Muriithi B., Okal M., Masiga D., Wanda G., Gisèle O., Samuel A., Nguertoum E., Nina R.A., Mansinsa P., Adam Y., Camara M., Olet P., Boucader D., Jamal S., Garba A.R.I., Ajakaiye J.J., Kinani J.F., Hassan M.A., Nonga H., Daffa J., Gidudu A., Chilongo K. The potential economic benefits of controlling trypanosomiasis using waterbuck repellent blend in sub-Saharan Africa. PLoS. One. 2021;16(7) doi: 10.1371/journal.pone.0254558. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

