Biased signaling via serotonin 5-HT2A receptor: From structural aspects to in vitro and in vivo pharmacology
- PMID: 41049744
- PMCID: PMC12491688
- DOI: 10.1016/j.apsb.2025.07.002
Biased signaling via serotonin 5-HT2A receptor: From structural aspects to in vitro and in vivo pharmacology
Abstract
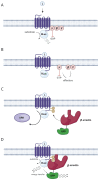
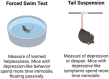
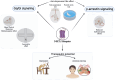
G protein-coupled receptors (GPCRs) represent key drug targets, with approximately 30%-40% of all medications acting on these receptors. Recent advancements have uncovered the complexity of GPCR signaling, including biased signaling, which allows selective activation of specific intracellular pathways-primarily mediated by G proteins and β-arrestins. Among aminergic GPCRs, the serotonin 5-HT2A receptor has garnered attention for its potential to generate therapeutic effects without adverse outcomes, such as hallucinations, through biased agonism. This review delivers a comprehensive overview of 5-HT2A receptor-biased signaling and its significance in developing safer mental health therapeutics, particularly for depression and anxiety. We provide a critical evaluation of methodologies for assessing biased signaling, spanning from traditional radioligand binding assays to advanced biosensor technologies. Furthermore, we review structural studies and computational modeling that have identified key receptor residues modulating biased signaling. We also highlight novel biased ligands with selective pathway activation, presenting a promising avenue for developing targeted antidepressant therapies without psychedelic effects. Additionally, we explore the 5-HT2A receptor's role in memory processes and stress response regulation. Ultimately, advancing our understanding of 5-HT2A receptor-biased signaling could drive the development of next-generation GPCR-targeted therapies, maximizing therapeutic efficacy while minimizing side effects in psychiatric treatment.
Keywords: 5-HT2A receptor; Biased agonism; Biased signaling; G protein-coupled receptors; Mental health disorders; Psychedelics.
© 2025 The Authors.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Tautermann C.S. GPCR structures in drug design, emerging opportunities with new structures. Bioorg Med Chem Lett. 2014;24:4073–4079. - PubMed
-
- Gray-Keller M.P., Detwiler P.B., Benovic J.L., Gurevich V.V. Arrestin with a single amino acid substitution quenches light-activated rhodopsin in a phosphorylation-independent fashion. Biochemistry. 1997;36:7058–7063. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

