Prior Authorization, Quantity Limits, and Step Therapy for Patient-Administered Antiemetics
- PMID: 41051776
- PMCID: PMC12501812
- DOI: 10.1001/jamanetworkopen.2025.35707
Prior Authorization, Quantity Limits, and Step Therapy for Patient-Administered Antiemetics
Abstract
Importance: Antiemetics have level 1 evidence supporting their role in improving quality of life and treatment tolerability for patients with cancer; they are an essential part of quality care. Utilization management (prior authorization, step therapy, and quantity limits) is implemented by insurance companies to improve effectiveness and efficiency and to control costs.
Objective: To characterize utilization management policies for antiemetics for Patient Protection and Affordable Care Act (ACA) Marketplace and Medicaid plans.
Design, setting, and participants: This cross-sectional study used a national sample of all available Medicaid and ACA drug formularies in 2024 linked to the US Food and Drug Administration's National Drug Codes. Formulations of the 13 most common self-administered (oral, sublingual, transdermal) antiemetics were identified.
Exposure: Plans' prior authorization, quantity limit, and step therapy policies.
Main outcomes and measures: The share of antiemetics subject to utilization management by coverage type (ACA, Medicaid, Medicaid managed care, state Medicaid), generic vs brand, and geographically by state was calculated.
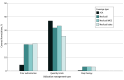
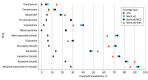
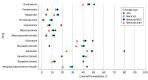
Results: A total of 561 formularies (301 [53.7%] ACA; 260 [46.3%] Medicaid) were included, amounting to 348 215 unique drug-plan formulations (173 607 [49.9%] ACA; 174 608 [50.1%] Medicaid). Overall, utilization management affected 66 981 covered antiemetic medications in ACA plans (39.7%) and 75 727 in Medicaid plans (43.4%). In ACA plans, a greater proportion of generic formulations faced restrictions than brand-name drugs (40.5% of generic [67 931 of 167 587] vs 17.4% of brand [1050 of 6020]). In Medicaid, this was the opposite: 82.5% of brand drugs (4357 of 5280) vs 42.2% of generic drugs (71 370 of 169 328) faced restrictions. Utilization management policies varied greatly by plan and state; some plans imposed at least 1 coverage restriction on 100% of antiemetic formulations, and others none. Quantity limits were the most prevalent utilization management tool, applying to more than one-third of covered antiemetic formulations in ACA (64 198 [37.0%]) and Medicaid plans (55 585 [38.1%]).
Conclusions and relevance: In this cross-sectional study of Medicaid and ACA formularies, there was substantial variation in utilization management requirements for antiemetics across insurance type and state as well as drug type, use, and class. Efforts to increase standardization of utilization management policies may alleviate administrative burden for patients.
Conflict of interest statement
Figures


References
-
- American Medical Association . 2023. AMA prior authorization physician survey. Accessed July 22, 2024. https://www.ama-assn.org/system/files/prior-authorization-survey.pdf
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

