Scutellarin suppresses Mycobacterium tuberculosis-induced pyroptosis in macrophages by inhibiting the HIF-1α-mediated Warburg effect
- PMID: 41051976
- PMCID: PMC12502121
- DOI: 10.1080/13510002.2025.2565861
Scutellarin suppresses Mycobacterium tuberculosis-induced pyroptosis in macrophages by inhibiting the HIF-1α-mediated Warburg effect
Abstract
Background: Mycobacterium tuberculosis (Mtb), the causative agent of tuberculosis (TB), remains a major global health threat due to prolonged treatment and drug-resistant strains. Host-directed therapy (HDT), which modulates host-pathogen interactions, offers potential to shorten treatment and limit resistance. This study investigates the effects of Scutellarin (SCU), a flavonoid from Scutellaria baicalensis, on Mtb-infected macrophages within the HDT framework.
Methods: Anti-pyroptotic and anti-inflammatory effects of SCU were assessed in Mtb-infected THP-1 and J774A.1 macrophages, and in a lipopolysaccharide (LPS)-induced acute lung injury (ALI) mouse model. Mitochondrial function was evaluated by oxygen consumption rate(OCR), membrane potential, and superoxide levels; glycolytic activity was measured by proton efflux rate (GlycoPER). Expression of inflammasome-related markers was analyzed by Western blot, qPCR, ELISA, immunofluorescence, and flow cytometry. The role of hypoxia-inducible factor 1-alpha (HIF-1α) was examined via siRNA knockdown.
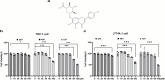
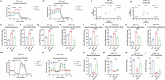
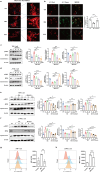
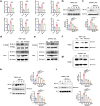
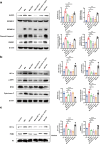
Results: SCU inhibited NLRP3 inflammasome activation, reduced IL-1β and IL-18 secretion, and attenuating pyroptosis. It restored mitochondrial integrity by regulating p-DRP1, MFN2, and Cytochrome C expression, and suppressed HIF-1α-mediated glycolytic reprogramming. Silencing of HIF-1α confirmed its role in SCU's mechanism. In vivo, SCU reduced pulmonary inflammation and cytokine release in LPS-induced ALI.
Conclusion: SCU alleviates Mtb-induced pyroptosis and inflammation in macrophages by inhibiting the HIF-1α-mediated Warburg effect.
Keywords: Mitochondrial dysfunction; Mycobacterium tuberculosis; NLRP3 inflammasome; Pyroptosis; Scutellarin; Warburg effect; host-directed therapy; hypoxia-inducible factor-1α.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


References
-
- Nahid P, Dorman SE, Alipanah N, et al. Executive summary: official American thoracic society/centers for disease control and prevention/Infectious Diseases Society of America clinical practice guidelines: treatment of drug-susceptible tuberculosis [J]. Clin Infect Dis. 2016;63(7):853–867. doi: 10.1093/cid/ciw566 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
