Pannexins in the vasculature
- PMID: 41051983
- PMCID: PMC12560215
- DOI: 10.1152/ajpheart.00510.2025
Pannexins in the vasculature
Abstract
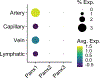
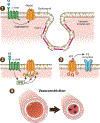
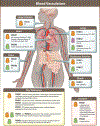
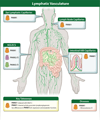
Pannexins (PANX1, PANX2, PANX3) are a family of large-pore, ion and metabolite channels present throughout the blood and lymphatic vascular networks. PANX1 has near-ubiquitous expression in the cardiovascular system and is the most highly studied pannexin in both homeostatic and disease conditions. In smooth muscle, endothelium, and blood cells, PANX1 acts at the cell surface as an ATP efflux channel to drive many vascular processes such as vasoconstriction, blood pressure, endothelial barrier function, platelet aggregation, and acute hypoxic responses. Conversely, PANX2 and PANX3 are understudied and exhibit a more intracellular localization pattern, with endothelial PANX3 modulating blood pressure through channel-independent mechanisms. In this review, we discuss the cellular localization and function of pannexins throughout the cardiovascular system, including resistance arteries, veins, lymphatics, large vessels, erythrocytes, platelets, pericytes, hearts, and lungs, as well as how this cellular activity corresponds to vascular physiology at the organism level. We also discuss the contribution of pannexins to the development and progression of various cardiovascular diseases, such as hypertension, edema, sepsis, atherosclerosis, aortic aneurysms, myocardial infarction, ischemia reperfusion, and thrombosis. In most cardiovascular diseases, PANX1 exacerbates disease development and progression, as evidenced by PANX1 channel blockade or genetic deletion in murine models improving disease outcomes, whereas the beneficial action of PANX3 in healthy vessels seems to be lost in conditions such as hypertension. With the prevalence of cardiovascular diseases and the associated burden on patients and healthcare systems, pannexin-based therapeutics may represent a novel alternative or combinatorial strategy for the treatment of many vascular conditions.
Keywords: arteries; endothelial cells; inflammation; ischemia reperfusion; smooth muscle cells.
Conflict of interest statement
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


References
-
- Michalski K, Syrjanen JL, Henze E, Kumpf J, Furukawa H, and Kawate T. The Cryo-EM structure of pannexin 1 reveals unique motifs for ion selection and inhibition. Elife 9: 2020. doi: 10.7554/eLife.54670. - DOI
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL120840/HL/NHLBI NIH HHS/United States
- R01 HL171997/HL/NHLBI NIH HHS/United States
- 165143/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- TL1 DK132771/DK/NIDDK NIH HHS/United States
- U2C DK129500/DK/NIDDK NIH HHS/United States
- U2CDK129500/HHS | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
- R01 HL137112/HL/NHLBI NIH HHS/United States
- 137112/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- T32 HL007284/HL/NHLBI NIH HHS/United States
- 120840/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- TL1DK132771/HHS | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
LinkOut - more resources
Full Text Sources

