Ibrutinib and rituximab versus immunochemotherapy in patients with previously untreated mantle cell lymphoma (ENRICH): a randomised, open-label, phase 2/3 superiority trial
- PMID: 41052510
- PMCID: PMC12549478
- DOI: 10.1016/S0140-6736(25)01432-1
Ibrutinib and rituximab versus immunochemotherapy in patients with previously untreated mantle cell lymphoma (ENRICH): a randomised, open-label, phase 2/3 superiority trial
Abstract
Background: Ibrutinib, a Bruton tyrosine kinase inhibitor, prolongs progression-free survival when added to immunochemotherapy as first line treatment. The ENRICH trial compared the chemotherapy-free combination of ibrutinib and the anti-CD20 antibody rituximab (ibrutinib-rituximab) with standard immunochemotherapy (R-CHOP [rituximab-cyclophosphamide, doxorubicin, vincristine, and prednisolone] or bendamustine-rituximab) in patients 60 years and older with untreated mantle-cell lymphoma.
Methods: This randomised, open-label, phase 2/3 superiority trial was performed at 66 sites in the UK, Sweden, Norway, Finland, and Denmark. Patients 60 years and older with untreated mantle-cell lymphoma (Ann-Arbor stage II-IV disease, an Eastern Cooperative Oncology Group performance-status score of 0-2) were randomly assigned to receive either rituximab plus immunochemotherapy or ibrutinib-rituximab in a 1:1 ratio, stratified by investigator choice of immunochemotherapy. Patients randomly allocated to the ibrutinib-rituximab (intervention) group received 560 mg oral ibrutinib daily in combination with six to eight cycles of 375 mg/m2 intravenous rituximab on day 1 of each cycle in the matched schedule of the pre-randomisation choice of immunochemotherapy (every 21 days for R-CHOP or every 28 days for rituximab-bendamustine). R-CHOP comprised 750 mg/m2 of cyclophosphamide, 50 mg/m2 of doxorubicin, and 1·4 mg/m2 vincristine on day 1 of each 21-day cycle, with 100 mg prednisolone on days 1-5 of each cycle. Rituximab-bendamustine comprised 90 mg/m2 of bendamustine on days 1 and 2 of each cycle, in combination with 375 mg/m2 rituximab on day 1 of each cycle. All responding patients in both groups at the end of induction received maintenance rituximab administered every 8 weeks for 2 years, and patients allocated to the intervention group continued ibrutinib until disease progression or unacceptable toxicity. The primary outcome was investigator-assessed progression-free survival, stratified by immunochemotherapy choice and analysed in the intention-to-treat population. The trial was registered with EudraCT (2015-000832-13) and is closed for recruitment.
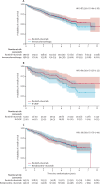
Findings: Between Feb 15, 2016, and June 30, 2021, 397 patients were randomly allocated to immunochemotherapy (control) or ibrutinib-rituximab (intervention). Of the 397, 107 (27%) were pre-allocated to the immunochemotherapy choice of R-CHOP and 290 (73%) were pre-allocated to rituximab-bendamustine. In total, 198 were allocated to the control group (53 to R-CHOP and 145 to bendamustine-rituximab) and 199 were allocated to intervention. The median age was 74 years (IQR 70-77) for the intervention group and 74 years (70-78) in the control group. 296 patients (75%) were male and 101 patients (25%) were female; ethnicity data were not collected. At a median follow-up of 47·9 months, the median progression-free survival of ibrutinib-rituximab was superior to immunochemotherapy, with an adjusted hazard ratio (HR) of 0·69 (95% CI 0·52-0·90); p=0·0034. For those with pre-randomisation choice R-CHOP, the HR was 0·37 (0·22-0·62), and with bendamustine-rituximab, the HR was 0·91 (0·66-1·25). Across induction and maintenance, 67% of patients assigned to ibrutinib-rituximab and 70% of patients receiving immunotherapy reported grade 3 or above adverse events.
Interpretation: To our knowledge, this is the first randomised trial in untreated mantle-cell lymphoma to demonstrate significant improvement in progression-free survival for ibrutinib-rituximab compared to immunochemotherapy. This study suggests that ibrutinib-rituximab should be considered a new standard of care option for first-line treatment of older patients with mantle-cell lymphoma.
Funding: Cancer Research UK (C7627/A17938) and Johnson and Johnson Pharmaceuticals.
Copyright © 2025 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests DJL reports consultancy and honoraria from AstraZeneca, Johnson and Johnson, Beigene, Roche, AbbVie, and Lilly. MJ reports honoraria from AbbVie, Johnson and Johnson, Roche, AstraZeneca, Kite (Gilead), and Lilly. IF reports consultancy fees from Kite (Gilead), Takeda, and Johnson and Johnson. CB reports consultancy and honoraria from Roche, Kite (Gilead), Takeda, Johnson and Johnson, AbbVie, and AstraZeneca. NC reports honoraria from AbbVie. MJB reports honoraria from Roche, Takeda, Celltrion, Kite (Gilead), Lilly, AbbVie, and Recordati; consulting or advisory roles for Incyte, Roche, Lilly, and AbbVie; and research funding, unrelated to the current work from Roche and Takeda. TAE reports consultancy and honoraria from Beigene, AstraZeneca, Roche, Gilead, Kite (Gilead), Takeda, Johnson and Johnson, Loxo Oncology, Incyte, Autolus, Nurix, and Galapagos. SR is a current employee of AstraZeneca. JR reports consultancy for Roche, AstraZeneca, and Kite (Gilead). AP reports consultancy or advisory roles for Beigene, AbbVie, Gilead, and Incyte. NM reports consultancy and honoraria from Takeda, Amgen, AbbVie, and Kite (Gilead). All other authors report no competing interests.
Figures




References
-
- Jain P, Wang M. High-risk MCL: recognition and treatment. Blood. 2025;145:683–695. - PubMed
-
- Robak T, Jin J, Pylypenko H, et al. Frontline bortezomib, rituximab, cyclophosphamide, doxorubicin, and prednisone (VR-CAP) versus rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) in transplantation-ineligible patients with newly diagnosed mantle cell lymphoma: final overall survival results of a randomised, open-label, phase 3 study. Lancet Oncol. 2018;19:1449–1458. - PubMed
-
- Kluin-Nelemans HC, Hoster E, Hermine O, et al. Treatment of older patients with mantle cell lymphoma (MCL): long-term follow-up of the randomized European MCL elderly trial. J Clin Oncol. 2020;38:248–256. - PubMed
-
- Eyre TA, Bishton MJ, McCulloch R, et al. Diagnosis and management of mantle cell lymphoma: a British Society for Haematology guideline. Br J Haematol. 2024;204:108–126. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

