Z-DNA-binding protein 1 exacerbates myocardial ischemia‒reperfusion injury by inducing noncanonical cardiomyocyte PANoptosis
- PMID: 41052986
- PMCID: PMC12501347
- DOI: 10.1038/s41392-025-02430-5
Z-DNA-binding protein 1 exacerbates myocardial ischemia‒reperfusion injury by inducing noncanonical cardiomyocyte PANoptosis
Abstract
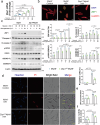
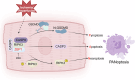
Myocardial ischemia‒reperfusion (I/R) injury is the primary factor that counteracts the beneficial effects of reperfusion therapy. Cardiomyocyte death serves as the fundamental pathological hallmark of I/R injury. However, targeting a single type of cell death has been reported to be ineffective at preventing I/R injury. ZBP1 is well established as a nucleic acid sensor that activates inflammatory and various cell death signaling pathways. However, the specific role of ZBP1 in adult cardiomyocytes, particularly in the absence of nucleic acid ligands, remains largely unexplored. In this study, our dynamic transcriptomic analyses at various I/R stages revealed a cluster of genes significantly enriched in cell death-related processes, with ZBP1 showing significant expression changes in both our I/R injury mouse model and public human ischemic cardiomyopathy datasets. Cardiomyocytes are the primary cell type expressing ZBP1 in response to I/R injury. Hypoxia/reoxygenation stress induced the upregulation of multiple cell death markers indicative of PANoptosis in adult cardiomyocytes, which was mitigated by ZBP1 deficiency. Compared with treatment with conventional cell death inhibitors, cardiomyocyte-specific Zbp1 deficiency ameliorated I/R-induced PANoptosis, resulting in a more substantial reduction in myocardial infarct size. Conversely, myocardial Zbp1 overexpression in adult mice directly induced cardiac remodeling and heart failure. Mechanistically, ZBP1 drives cardiomyocyte PANoptosis by promoting the formation of the ZBP1/RIPK3/CAS8/CAS6 PANoptosome complex. Virtual screening and experimental validation revealed a novel small-molecule compound, MSB, which has high binding affinity for ZBP1 and effectively attenuates myocardial I/R injury both in vitro and in vivo. Collectively, these findings highlight the role of ZBP1 as a mediator of cardiomyocyte PANoptosis and suggest that targeting ZBP1 could be a promising strategy for mitigating myocardial I/R injury.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

