Daratumumab plus lenalidomide maintenance in newly diagnosed multiple myeloma after transplant: AURIGA subgroup analyses
- PMID: 41053004
- PMCID: PMC12500855
- DOI: 10.1038/s41408-025-01355-0
Daratumumab plus lenalidomide maintenance in newly diagnosed multiple myeloma after transplant: AURIGA subgroup analyses
Abstract
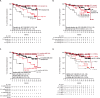
In the primary analysis (32.3-month median follow-up) of the randomized, phase 3 AURIGA study (NCT03901963), daratumumab-lenalidomide (D-R) maintenance significantly improved MRD-negative conversion rates and reduced the risk of disease progression or death by 47% versus R maintenance in anti-CD38 monoclonal antibody-naïve and post-transplant MRD-positive patients with newly diagnosed MM. Here, we present a post hoc analysis across relevant subgroups, including high-risk cytogenetic abnormalities (HRCAs) per original, revised, and modified International Myeloma Society (IMS) 2024 criteria. MRD-negative (10-5) conversion rates by 12 months of maintenance were higher for D-R versus R across cytogenetically high-risk subgroups per original (31.8% vs 6.7%), revised (43.8% vs 13.3%), and modified IMS 2024 (41.2% vs 0%) criteria and cytogenetically ultra-high-risk disease (≥2 revised HRCAs; 54.5% vs 0%). Similar trends in overall MRD-negative conversion rates were observed across subgroups. D-R demonstrated a trend towards improved PFS versus R (HR [95% CI]) in cytogenetically high-risk subgroups per original (0.60 [0.21-1.70]), revised (0.53 [0.21-1.31]), and modified IMS 2024 (0.45 [0.13-1.53]) criteria and cytogenetically ultra-high-risk disease (0.61 [0.17-2.25]). Similar outcomes were observed regardless of age or race, with no additional safety concerns among older (≥65 years) or Black patients. These data support the benefit of D-R maintenance regardless of age, race, and risk status.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: LF served on advisory boards and as a site principal investigator for Bristol Myers Squibb, Johnson & Johnson, and Pfizer. LDA served as a consultant and on advisory boards for Johnson & Johnson, Celgene, Bristol Myers Squibb, Amgen, GSK, AbbVie, BeiGene, Cellectar Biosciences, Sanofi, and Prothena; served on the data safety monitoring board for Prothena; and received institutional research funding from Bristol Myers Squibb, AbbVie, Regeneron, Caelum, CARsgen, Cellectar Biosciences, AstraZeneca, Arcellx, and Johnson & Johnson. A Chung served as a consultant and on an advisory board for Bristol Myers Squibb and Johnson & Johnson; and received institutional research funding from AbbVie, Bristol Myers Squibb, Caelum, CARsgen, Cellectis, Johnson & Johnson, K36 Therapeutics, and Merck. CPC received honoraria from Johnson & Johnson and Sanofi/Genzyme. EP has nothing to declare. AJC served as a consultant or in an advisory role for Sebia, Johnson & Johnson, Bristol Myers Squibb, Sanofi, HopeAI, Adaptive Biotechnologies, and AbbVie; and received institutional research funding from Johnson & Johnson, Bristol Myers Squibb, Juno/Celgene, Sanofi, Regeneron, IGM Biosciences, Nektar, Harpoon, and Caelum. CC served as a consultant for Bristol Myers Squibb, Johnson & Johnson, Pfizer, Karyopharm, and Genentech; and received institutional research funding from Bristol Myers Squibb, Johnson & Johnson, Takeda, Ionis, Poseida, and Harpoon. SL received institutional research funding from Johnson & Johnson, Allogene, Bioline, Pfizer, Bristol Myers Squibb, Regeneron, Sanofi, Ionis, and ImmPACT Bio; and owns stock or stock options for TORL BioTherapeutics. DWS served as a consultant or in an advisory role for GSK, Johnson & Johnson, Sanofi, AbbVie, Bristol Myers Squibb, Pfizer, Opna Bio, Arcellx, AstraZeneca, and Genentech; and received institutional research funding from Pfizer. KHS served on an advisory board for Bristol Myers Squibb, Johnson & Johnson, Sanofi, and GSK; received institutional research funding from AbbVie and Karyopharm; and received honoraria from Karyopharm, Johnson & Johnson, Adaptive Biotechnologies, GSK, Bristol Myers Squibb, Sanofi/Genzyme, and Regeneron. RS served as a consultant or in an advisory role for Sanofi, Johnson & Johnson, and Oncopeptides; and received institutional research funding from Sanofi. PV served as a consultant for, received honoraria from, and holds a membership on an entity’s board of directors or advisory committees for AbbVie, Bristol Myers Squibb, Karyopharm, Regeneron, and Sanofi. MK, HP, SP, VK, RC, and TSL are employees of and may hold stock in Johnson & Johnson. A Cortoos is a former employee of and may hold stock in Johnson & Johnson. AB received research funding from Bristol Myers Squibb, GSK, BeiGene, Roche, and Johnson & Johnson.
Figures

References
-
- de Weers M, Tai YT, van der Veer MS, Bakker JM, Vink T, Jacobs DC, et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol. 2011;186:1840–8. - PubMed
-
- Lammerts van Bueren J, Jakobs D, Kaldenhoven N, Roza M, Hiddingh S, Meesters J, et al. Direct in vitro comparison of daratumumab with surrogate analogs of CD38 antibodies MOR03087, SAR650984 and Ab79. Blood. 2014;124:3474.
-
- Overdijk MB, Jansen JH, Nederend M, Lammerts van Bueren JJ, Groen RW, Parren PW, et al. The therapeutic CD38 monoclonal antibody daratumumab induces programmed cell death via Fcgamma receptor-mediated cross-linking. J Immunol. 2016;197:807–13. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

