Hepatic steatosis and pyroptosis are induced by the hepatitis B virus X protein via B56α-METTL3 interaction-mediated m6A modification of the NLRP3 mRNA
- PMID: 41053025
- PMCID: PMC12500908
- DOI: 10.1038/s41419-025-08019-8
Hepatic steatosis and pyroptosis are induced by the hepatitis B virus X protein via B56α-METTL3 interaction-mediated m6A modification of the NLRP3 mRNA
Abstract
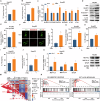
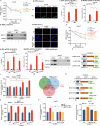
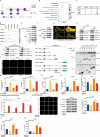
Metabolic dysfunction-associated steatohepatitis (MASH) is one of the fastest-growing chronic liver diseases and is characterized by excessive steatosis, inflammation, and progressive liver injury. The hepatitis B virus (HBV) X protein (HBx) is a major viral factor that contributes to the onset and progression of MASH. Emerging evidence highlights the role of epigenetic modifications, particularly N6-methyladenosine (m6A), as prevalent modifications of mRNAs that play crucial roles in MASH pathogenesis by regulating mRNA stability, translation, processing, and nuclear export. However, the epigenetic mechanisms by which m6A modification contributes to HBx-related MASH remain poorly defined. In this study, we observed that NOD-like receptor protein 3 (NLRP3)-dependent pyroptosis and intracellular lipid accumulation are markedly elevated in the livers of HBx-transgenic (HBx-Tg) mice in vivo and in HBx-expressing hepatocytes in vitro, exacerbating liver injury and driving MASH progression. Integrated metabolomic and transcriptomic analyses of HBx-Tg mice revealed distinct gene expression alterations, suggesting a key role for m6A modification in mediating hepatic inflammation and lipotoxicity. Mechanistically, we identified methyltransferase-like 3 (METTL3) as a critical positive regulator of this process. HBx upregulated METTL3 expression and the m6A level of NLRP3 mRNA in HBx-expressing hepatocytes, whereas METTL3 knockdown or catalytic inactivation suppressed NLRP3-dependent pyroptosis. Further investigation revealed that METTL3 enhances NLRP3 mRNA stability via m6A modification at A2748 site in the coding sequence. Moreover, the protein phosphatase 2A (PP2A) B56α subunit was found to interact with the METTL3 methyltransferase domain (MTD), facilitating its enzymatic activity and further increasing NLRP3 m6A methylation, thereby promoting pyroptosis and lipid accumulation in HBx-expressing hepatocytes. Importantly, treatment with STM2457, a selective inhibitor targeting the METTL3 MTD, significantly attenuated hepatic inflammation, steatohepatitis, and lipotoxicity. Taken together, our findings advance the understanding of HBx-induced hepatic lipid accumulation, steatosis, inflammasome formation, and pyroptosis, and indicate that targeting METTL3 with STM2457 intervention is a promising approach for MASH treatment.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics: All methods were approved by the Ethical Committee for Animal Experimentation at Xiamen University (Ethics Approval No. XMULAC20220282, dated 2022-03-12) and conducted following the Guide for the Care and Use of Laboratory Animals. This study was conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from all participants for the use of tissue samples and clinical data in experiments and for publication.
Figures




References
-
- Riazi K, Azhari H, Charette JH, Underwood FE, King JA, Afshar EE, et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2022;7:851–61. - PubMed
-
- Targher G, Byrne CD, Tilg H. MASLD: a systemic metabolic disorder with cardiovascular and malignant complications. Gut. 2024;73:691–702. - PubMed
-
- Nie Q, Luo X, Wang K, Ding Y, Jia S, Zhao Q, et al. Gut symbionts alleviate MASH through a secondary bile acid biosynthetic pathway. Cell. 2024;187:2717–34 e33. - PubMed
-
- Bansal SK, Bansal MB. Pathogenesis of MASLD and MASH - role of insulin resistance and lipotoxicity. Aliment Pharm Ther. 2024;59:S10–S22. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

