Neutralizing and binding antibodies are a correlate of risk of COVID-19 in the CoVPN 3008 study in people with HIV
- PMID: 41053137
- PMCID: PMC12501024
- DOI: 10.1038/s41467-025-63948-4
Neutralizing and binding antibodies are a correlate of risk of COVID-19 in the CoVPN 3008 study in people with HIV
Abstract
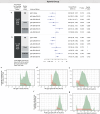
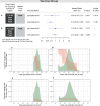
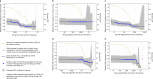
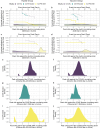
People with HIV (PWH) are understudied in COVID-19 vaccine trials, leaving knowledge gaps on whether the identified immune correlates of protection also hold in PWH. CoVPN 3008 (NCT05168813) enrolled predominantly PWH and reported lower COVID-19 incidence for a Hybrid vs. Vaccine Group (baseline SARS-CoV-2-positive and one mRNA-1273 dose vs. negative and two doses). Using case-cohort sampling, antibody markers at enrolment (M0) and four weeks post-final vaccination (Peak) are assessed as immune correlates of COVID-19. For the Hybrid Group [n = 287 (195 PWH)], all M0 markers inversely correlate with COVID-19 through 230 days post-Peak, with 50% inhibitory dilution BA.4/5 neutralizing antibody titer (nAb-ID50 BA.4/5) the strongest and only independent correlate (HR per 10-fold increase=0.46, 95% CI 0.28, 0.75; P = 0.002). For the Vaccine Group [n = 115 (86 PWH)], Peak nAb-ID50 BA.4/5 correlates with reduced COVID-19 risk (1.9%, 1.1%, and 0.3% at titers 10, 100, and 1000 AU/ml) through 92, but not 165, days post-Peak. Using multivariable Cox analysis of binding and nAb, nAb titers predict COVID-19 in PWH. Two doses of a 100-µg Ancestral strain mRNA vaccine in baseline-SARS-CoV-2-negative individuals elicit sufficient cross-reacting Omicron antibodies to reduce COVID-19 incidence for 90 days post-Peak, but viral evolution and waning antibodies abrogate this protection thereafter.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: L.N.C. was compensated for work on this manuscript through a consulting agreement with Fred Hutchinson Cancer Center. L.C., F.L., S.B.-F., S.D.-M., M.C.H., C.A.M., E.A.-N., Y.H., M.J.M., G.M., N.M.M., P.L.M., and J.A. received funding from NIAID/NIH paid to their institutions. S.D. reports salary support from Johns Hopkins University. M.J.M. reports NIAID and Bill & Melinda Gates Foundation payments to her institution and payment from Stanford, CROI, NIH VRC, and the Ragon Institute. P.B.G. received funds from NIAID/NIH paid to his institution, consulting fees from Curevo Vaccine Company and MinervaX Vaccine Company, served unpaid on a Moderna Advisory Board for Zika vaccines, and received contracts to his institution for AstraZeneca Vaccine SAB, Sanofi SAB, and Vaccine Company SAB. All other authors have nothing to declare.
Figures


References
-
- El Chaer, F. & El Sahly, H. M. Vaccination in the adult patient infected with HIV: a review of vaccine efficacy and immunogenicity. Am. J. Med132, 437–446 (2019). - PubMed
MeSH terms
Substances
Grants and funding
- R37AI054165/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- S10 OD028685/OD/NIH HHS/United States
- T32 AI007044/AI/NIAID NIH HHS/United States
- UM1 AI068614-14/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- T32AI007044-45/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- UM1 AI068618/AI/NIAID NIH HHS/United States
- R37 AI054165/AI/NIAID NIH HHS/United States
- UM1 AI068635/AI/NIAID NIH HHS/United States
- UM1 AI068619/AI/NIAID NIH HHS/United States
- UM1 AI068614/AI/NIAID NIH HHS/United States
- 3UM1AI068618-15S1/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

