Comparison of effect of massage or chicory bath on bilirubin levels in term newborns undergoing phototherapy: a randomized clinical trial
- PMID: 41053652
- PMCID: PMC12502538
- DOI: 10.1186/s12887-025-06170-x
Comparison of effect of massage or chicory bath on bilirubin levels in term newborns undergoing phototherapy: a randomized clinical trial
Abstract
Background: Neonatal hyperbilirubinemia is a frequent metabolic complication in newborns. The available treatment methods may bring about adverse consequences; therefore, finding new modalities to decrease the duration of treatment is a major concern. The aim of this study was to compare the effect of massage or chicory bath on bilirubin levels in term newborns undergoing phototherapy.
Methods: This randomized clinical trial study was conducted on 90 newborns with hyperbilirubinemia at Imam Ali Hospital of Amol on October 26, 2024 to February 6, 2025. Two intervention groups received chicory bath or massage as an adjuvant intervention in addition to standard phototherapy treatment, and the control group only received phototherapy. A custom-designed data collection form was used in this study. All newborns who met the inclusion criteria were selected and informed consent was obtained from their parents. Then, using a computerized random number table, the newborns were randomly assigned to three groups. Sampling continued until the intended number was reached. Descriptive and analytical statistical tests including analysis of variance, chi-square, Tukey's post hoc test, and repeated measures ANOVA were used to analyze the data using SPSS software (version 26). A significance level of 0.05 was considered acceptable.
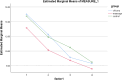
Results: There was no significant difference between the three groups in terms of neonatal age, gender, birth weight, gestational age, and type of delivery. There was no significant difference between the three groups regarding average bilirubin levels on the second, third, and fourth days of hospitalization. The change in bilirubin levels over time was significant regardless of the group (P=0.000), and the trend of bilirubin change over time was not significant among the three groups. There was no significant difference in the rate of complications including skin rashes, drug sensitivity, and phototherapy sensitivity among the three groups. There was a significant difference between the three groups in terms of the frequency of defecation on the third and fourth days (P=0.02), which was higher in the massage group. There was a statistically significant difference in the number of hospitalization days among the chicory (3 ±0. 91) (Length of stay: 2-5), massage (2.77±0.73) (Length of stay: 2-4), and control groups (3.37±0.93) (Length of stay: 2-5) (p=0.03). This significant difference was observed between massage and control groups (P=0.02) concerning the rate of hospitalization, and the newborns in the massage group were discharged earlier.
Conclusion: Based on the results of the present study, Field massage combined with phototherapy was more effective in increasing the frequency of defecation and reducing the length of hospitalization in infants with hyperbilirubinemia compared to those in the chicory bath group and the control group who received phototherapy only.
Trial registration: This trial was registered on https://irct.behdasht.gov.ir (trial ID 77302).
Keywords: Bath; Chicory; Jaundice; Massage; Newborn.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Research Ethical Committee of Mazandaran University of Medical Sciences in Iran approved this medical research project using the ethical code: IR.MAZUMS.REC.1403.180. Informed consent was obtained from the parents of all participants before their inclusion in the study. All study methods were implemented in line with the detailed guidelines of the Helsinki Declaration. Consent for publication: Informed consent for the publication of data was taken from new born’ guardians and all the data can be published. Competing interests: The authors declare no competing interests.
Figures
References
-
- Maisels MJ, McDonagh AF. Phototherapy for neonatal jaundice. N Engl J Med. 2008;358(9):920. 8.[ 10.1056/NEJMct0708376]. - PubMed
-
- Behjati S, Sagheb S, Aryasepehr S, Yaghmai B. Adverse events associated with neonatal exchange transfusion for hyperbilirubinemia. Indian J Pediatr. 2009;76(1):83–5. 10.1007/s12098-009-0033-1]. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous