Laboratory evaluation of 185 commercial assays for detecting SARS-CoV-2: the UK response for mass testing
- PMID: 41054442
- PMCID: PMC12496213
- DOI: 10.1016/j.eclinm.2025.103416
Laboratory evaluation of 185 commercial assays for detecting SARS-CoV-2: the UK response for mass testing
Abstract
Background: In August 2020, Public Health England and Oxford University were commissioned to design and deliver (with NHS Test and Trace, NHSTT) a rapid evaluation programme of antigen Lateral Flow Devices (LFDs) for SARS-CoV-2 for mass community testing.
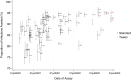
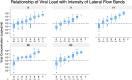
Methods: A three-phase evaluation process was established: 1) desktop review of kits including claimed performance and supply; 2) laboratory testing with laboratory-grown SARS-CoV-2 virus and SARS-CoV-2 virus PCR negative volunteer samples; and 3) larger-scale laboratory testing of SARS-CoV-2 PCR positive and negative clinical samples. Variant of Concern (VOC) identification in the UK (December 2020), expanded laboratory methodology. Processes also evolved to improve workflow (irradiated viral stocks, dilution matrices, sample volumes, and replicates).
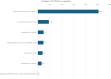
Findings: Overall, 1017 kits were screened at phase 1, 185 kits tested at phase 2 and 91 at phase 3. Sixteen kits failed phase 3 due to poor performance and eight more failed to detect VOC satisfactorily. Sixty-four kits were redesigns of previously failed kits. The overall pass rate for the laboratory evaluation was 35% and 5 kits were procured for the UK National Covid 19 Testing Programme.
Interpretation: The evaluation results had potential, time limited commercially sensitive aspects, and public sharing was limited to kits passing phase 3. Until now, the full data set has not been published. Over 2.5 billion self-test kits were deployed by the UK government following purchasing decisions informed by this work. We offer a potential blueprint for future evaluation programmes that might be required to assess LFDs to detect cases of a pandemic novel pathogen.
Funding: This study was funded by UK Department of Health and Social Care (UKHSA); UK Health Security Agency (formerly Public Health England and the National Health Service Test and Trace); and the University of OxfordNIHRBiomedical Research Centre.
Keywords: Coronavirus; LFD; Laboratory evaluation; Lateral flow device; Mass-testing; Pandemic; Public health; SARS-CoV-2.
Crown Copyright © 2025 Published by Elsevier Ltd.
Conflict of interest statement
SHo is supported by grants from MRC and NIHR. TP was Membership of National Lateral Flow Test Advisory Committee. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, the Department of Health and Social Care (DHSC) or the UKHSA.
Figures

References
-
- Public Health England (PHE) PHE; London: 2021. COVID-19:laboratory evaluations of serological assays.https://www.gov.uk/government/publications/covid-19-laboratory-evaluatio...
LinkOut - more resources
Full Text Sources
Miscellaneous

