Advance care planning in multiple sclerosis (ConCure-SM): A multicenter single-arm pilot and feasibility study
- PMID: 41056241
- PMCID: PMC12503263
- DOI: 10.1371/journal.pone.0331220
Advance care planning in multiple sclerosis (ConCure-SM): A multicenter single-arm pilot and feasibility study
Abstract
Background: Advance care planning (ACP) practice in people with progressive multiple sclerosis (PwPMS) remains limited. ConCure-SM project aims to assess the effectiveness of a structured ACP intervention (clinician's training programme and use of a booklet during ACP conversations) using a multi-phased design.
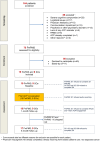
Methods: Single-arm pilot/feasibility trial involving PwPMS, their significant others (SOs), and clinicians from six Italian centers. Primary study outcome was completion of an advance care plan document (ACP-Doc). Other outcomes included safety, feasibility of enrollment and assessment, and (analyzed using mixed-methods approach) Hospital Anxiety and Depression Scale (HADS), quality of communication, quality of life (MSQOL-29), and caregiver burden. Participants were interviewed to identify factors influencing the ACP implementation process.
Results: Seventy-five PwPMS were eligible out of 164 screened; 56/75 (75%) refused participation and 19 were included. Of these, 11 (58% vs 30% hypothesized) completed the ACP-Doc. A total of 25 adverse events (increase in anxiety) occurred, three possibly related to the intervention, and we found a worsening of HADS-Anxiety score (p = 0.02) and MSQOL-29 mental health composite score (p = 0.04) during follow-up. PwPMS/SO interviews revealed four themes: significance of the ACP process (on the individual, on relation with clinicians), its impact (on emotions, on family relations), preparedness as key, and challenges (practicability, SO commitment). Barriers and facilitators for ACP were identified in two clinician focus groups.
Conclusions: The intervention supported neurologists in guiding PwPMS in their ACP. However, trial findings and the high proportion of refusals point to the need to enrich the intervention with a new component targeting PwPMS and SOs.
Trial registration: ISRCTN48527663.
Copyright: © 2025 Solari et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
AL reports grants from Novartis, during the conduct of the study; personal fees from Biogen, Merck Serono, Mylan, Novartis, Roche, Sanofi/Genzyme, Teva and FISM. FP received personal compensation for serving on advisory board and/or speaking activities by Almirall, Bayer, Biogen, Bristol Meyers & Squibb, Merck, Novartis Roche, Sanofi and TEVA; he further received research grants by Biogen Italy, Biogen Global, Merck, University of Catania, FISM and Reload Onlus Patients Association. AS received personal compensation for serving on advisory board and speaking activities by Almirall and Merck. All the other authors have no competing interests. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures

References
-
- Rietjens JAC, Sudore RL, Connolly M, van Delden JJ, Drickamer MA, Droger M, et al. Definition and recommendations for advance care planning: an international consensus supported by the European Association for Palliative Care. Lancet Oncol. 2017;18(9):e543–51. doi: 10.1016/S1470-2045(17)30582-X - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

