Autoinducer-2-mediated communication network within human gut microbiota
- PMID: 41056492
- PMCID: PMC12503165
- DOI: 10.1093/ismejo/wraf204
Autoinducer-2-mediated communication network within human gut microbiota
Abstract
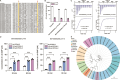
Quorum sensing (QS) is a chemical communication process that connects microbial members in various microbial systems. Bacterial communication networks mediated by QS play important roles in the regulation of intestinal microecological balance as well as nutrition and metabolism of the host. However, how human gut microbes utilize QS signals to communicate with one another remains largely unknown. In this study, we first examined the prevalence and abundance of genes encoding QS signal synthases in 3329 species representatives clustered from 289232 prokaryotic genomes in the Unified Human Gastrointestinal Genome collection. Our results show autoinducer-2 (AI-2) is the most prevalent QS signal within the human gut microbiota, with the synthase gene luxS being found in 2039 species mainly distributed within Firmicutes, Actinobacteriota, Bacteroidota, and Proteobacteria. Furthermore, 299 species carry genes encoding one or more types of AI-2 receptors (LuxP-, LsrB-, dCache_1-, and GAPES1-type). The dCache_1- and GAPES1-type receptors can function as methyl-accepting chemotaxis proteins, histidine kinases, c-di-GMP synthases and/or c-di-GMP-specific phosphodiesterases, serine phosphatases, and serine/threonine kinases, suggesting the diversity of AI-2-mediated interspecies communication modes among human gut microbiota. Metatranscriptomic analysis showed that a number of AI-2 synthase- and receptor-encoding genes can be expressed in the human gut in healthy and/or unhealthy states. The communication network analysis suggests that AI-2-mediated interactions widely occur among members of Firmicutes, Proteobacteria, Actinobacteriota, Campylobacterota, and Spirochaetota. Overall, this study deepens understanding of QS-mediated communication network among human gut microbiota, and provides guidance for engineering gut microbiota and constructing new synthetic microbial consortia based on complex microbial interactions.
Keywords: AI-2 receptors; Quorum sensing; autoinducer-2; communication network; human gut microbiota.
© The Author(s) 2025. Published by Oxford University Press on behalf of the International Society for Microbial Ecology.
Conflict of interest statement
None declared.
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

