Lower bounds for the Zagreb indices of trees with given total domination number and its applications in QSPR studies of alkanes
- PMID: 41057480
- PMCID: PMC12504606
- DOI: 10.1038/s41598-025-18870-6
Lower bounds for the Zagreb indices of trees with given total domination number and its applications in QSPR studies of alkanes
Abstract
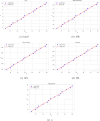
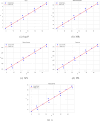
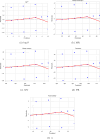
Understanding the relationship between molecular structure and physicochemical properties is a central problem in mathematical chemistry and molecular informatics. Among the many topological descriptors used for this purpose, Zagreb indices play a significant role due to their proven relevance in quantitative structure-property relationship (QSPR) studies. Motivated by the need for structural insight into molecules modeled as trees, this work focuses on deriving lower bounds for the first and second Zagreb indices of trees with a fixed total domination number. By analyzing the structural properties of such trees, we establish new inequalities that highlight the interplay between domination parameters and molecular descriptors. To validate their practical relevance, we apply the derived bounds in a QSPR context, specifically examining their correlation with key physicochemical properties of alkanes. The statistical analysis reveals strong predictive capability, with near-to-unity correlation coefficients between the computed bounds and experimental data. These results demonstrate the potential of domination-theoretic methods in advancing predictive modeling in chemical graph theory.
Keywords: Extremal trees; QSPR analysis; Total domination number; Zagreb indices.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures
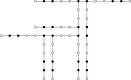
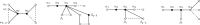

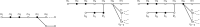
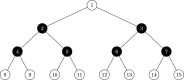

References
-
- Trinajstic, N. Chemical Graph Theory (CRC Press, Boca Raton, 2018).
-
-
Gutman, I. & Trinajstić, N. Graph theory and molecular orbitals. Total
 electron energy of alternant hydrocarbons. Chem. Phys. Lett.17(4), 535–538 (1972).
electron energy of alternant hydrocarbons. Chem. Phys. Lett.17(4), 535–538 (1972).
-
Gutman, I. & Trinajstić, N. Graph theory and molecular orbitals. Total
-
- Cockayne, E. J., Dawes, R. & Hedetniemi, S. T. Total domination in graphs. Networks10(3), 211–219 (1980).
-
- Das, K. C., Huh, D.-Y., Bera, J. & Mondal, S. Study on geometric-arithmetic, arithmetic-geometric and Randić indices of graphs. Discrete Appl. Math.360, 229–245. 10.1016/j.dam.2024.09.007 (2025).
-
- Mondal, S., Das, K. C. & Huh, D. The minimal chemical tree for the difference between geometric-arithmetic and Randić indices. Int. J. Quantum Chem.124(1), 27336. 10.1002/qua.27336 (2024).
LinkOut - more resources
Full Text Sources

